T-regulatory cell treatment prevents chronic rejection of heart allografts in a murine mixed chimerism model
- PMID: 24468120
- PMCID: PMC3991417
- DOI: 10.1016/j.healun.2013.11.004
T-regulatory cell treatment prevents chronic rejection of heart allografts in a murine mixed chimerism model
Abstract
Background: The mixed chimerism approach induces donor-specific tolerance in both pre-clinical models and clinical pilot trials. However, chronic rejection of heart allografts and acute rejection of skin allografts were observed in some chimeric animals despite persistent hematopoietic chimerism and tolerance toward donor antigens in vitro. We tested whether additional cell therapy with regulatory T cells (Tregs) is able to induce full immunologic tolerance and prevent chronic rejection.
Methods: We recently developed a murine "Treg bone marrow (BM) transplantation (BMT) protocol" that is devoid of cytoreductive recipient pre-treatment. The protocol consists of a moderate dose of fully mismatched allogeneic donor BM under costimulation blockade, together with polyclonal recipient Tregs and rapamycin. Control groups received BMT under non-myeloablative irradiation and costimulation blockade without Treg therapy. Multilineage chimerism was followed by flow cytometry, and tolerance was assessed by donor-specific skin and heart allografts.
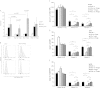
Results: Durable multilineage chimerism and long-term donor skin and heart allograft survival were successfully achieved with both protocols. Notably, histologic examination of heart allografts at the end of follow-up revealed that chronic rejection is prevented only in chimeras induced with the Treg protocol.
Conclusions: In a mouse model of mixed chimerism, additional Treg treatment at the time of BMT prevents chronic rejection of heart allografts. As the Treg-chimerism protocol also obviates the need for cytoreductive recipient treatment it improves both efficacy and safety over previous non-myeloablative mixed chimerism regimens. These results may significantly impact the development of protocols for tolerance induction in cardiac transplantation.
Keywords: chronic rejection; costimulation blockade; heart transplantation; mixed chimerism; regulatory T cells; tolerance.
© 2014 International Society for Heart and Lung Transplantation Published by International Society for the Heart and Lung Transplantation All rights reserved.
Figures


Similar articles
-
Combinations of anti-LFA-1, everolimus, anti-CD40 ligand, and allogeneic bone marrow induce central transplantation tolerance through hemopoietic chimerism, including protection from chronic heart allograft rejection.J Immunol. 2004 Dec 1;173(11):7025-36. doi: 10.4049/jimmunol.173.11.7025. J Immunol. 2004. PMID: 15557200
-
Effect of Ex Vivo-Expanded Recipient Regulatory T Cells on Hematopoietic Chimerism and Kidney Allograft Tolerance Across MHC Barriers in Cynomolgus Macaques.Transplantation. 2017 Feb;101(2):274-283. doi: 10.1097/TP.0000000000001559. Transplantation. 2017. PMID: 27846155 Free PMC article.
-
Therapeutic efficacy of polyclonal tregs does not require rapamycin in a low-dose irradiation bone marrow transplantation model.Transplantation. 2011 Aug 15;92(3):280-8. doi: 10.1097/TP.0b013e3182241133. Transplantation. 2011. PMID: 21697774
-
Chimerism-Based Tolerance to Kidney Allografts in Humans: Novel Insights and Future Perspectives.Front Immunol. 2022 Jan 5;12:791725. doi: 10.3389/fimmu.2021.791725. eCollection 2021. Front Immunol. 2022. PMID: 35069574 Free PMC article. Review.
-
Inducing mixed chimerism and transplantation tolerance through allogeneic bone marrow transplantation with costimulation blockade.Methods Mol Biol. 2007;380:391-403. doi: 10.1007/978-1-59745-395-0_25. Methods Mol Biol. 2007. PMID: 17876108 Review.
Cited by
-
IL-2/α-IL-2 Complex Treatment Cannot Be Substituted for the Adoptive Transfer of Regulatory T cells to Promote Bone Marrow Engraftment.PLoS One. 2016 Jan 5;11(1):e0146245. doi: 10.1371/journal.pone.0146245. eCollection 2016. PLoS One. 2016. PMID: 26731275 Free PMC article.
-
Cardiac endothelial cell-derived exosomes induce specific regulatory B cells.Sci Rep. 2014 Dec 23;4:7583. doi: 10.1038/srep07583. Sci Rep. 2014. PMID: 25533220 Free PMC article.
-
Creation of human hematopoietic chimeric cell (HHCC) line as a novel strategy for tolerance induction in transplantation.Stem Cell Investig. 2022 Dec 19;9:11. doi: 10.21037/sci-2022-026. eCollection 2022. Stem Cell Investig. 2022. PMID: 36619595 Free PMC article.
-
A heart transplant center experience with basiliximab induction strategies: A double edged sword?Clin Transplant. 2024 Apr;38(4):e15307. doi: 10.1111/ctr.15307. Clin Transplant. 2024. PMID: 38567897
-
Minor Antigen Disparities Impede Induction of Long Lasting Chimerism and Tolerance through Bone Marrow Transplantation with Costimulation Blockade.J Immunol Res. 2016;2016:8635721. doi: 10.1155/2016/8635721. Epub 2016 Oct 31. J Immunol Res. 2016. PMID: 27872868 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

