A role for sorting nexin 27 in AMPA receptor trafficking
- PMID: 24458027
- PMCID: PMC3921469
- DOI: 10.1038/ncomms4176
A role for sorting nexin 27 in AMPA receptor trafficking
Abstract
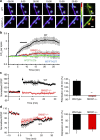
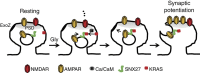
Sorting nexin 27 (SNX27), a PDZ domain-containing endosomal protein, was recently shown to modulate glutamate receptor recycling in Down's syndrome. However, the precise molecular role of SNX27 in GluA1 trafficking is unclear. Here we report that SNX27 is enriched in dendrites and spines, along with recycling endosomes. Significantly, the mobilization of SNX27 along with recycling endosomes into spines was observed. Mechanistically, SNX27 interacts with K-ras GTPase via the RA domain; and following chemical LTP stimuli, K-ras is recruited to SNX27-enriched endosomes through a Ca(2+)/CaM-dependent mechanism, which in turn drives the synaptic delivery of homomeric GluA1 receptors. Impairment of SNX27 prevents LTP and associated trafficking of AMPARs. These results demonstrate a role for SNX27 in neuronal plasticity, provide a molecular explanation for the K-ras signal during LTP and identify SNX27 as the PDZ-containing molecular linker that couples the plasticity stimuli to the delivery of postsynaptic cargo.
Figures
Similar articles
-
Loss of sorting nexin 27 contributes to excitatory synaptic dysfunction by modulating glutamate receptor recycling in Down's syndrome.Nat Med. 2013 Apr;19(4):473-80. doi: 10.1038/nm.3117. Epub 2013 Mar 24. Nat Med. 2013. PMID: 23524343 Free PMC article.
-
Sorting Nexin 27 regulates basal and activity-dependent trafficking of AMPARs.Proc Natl Acad Sci U S A. 2014 Aug 12;111(32):11840-5. doi: 10.1073/pnas.1412415111. Epub 2014 Jul 28. Proc Natl Acad Sci U S A. 2014. PMID: 25071192 Free PMC article.
-
Sorting nexin-27 regulates AMPA receptor trafficking through the synaptic adhesion protein LRFN2.Elife. 2021 Jul 12;10:e59432. doi: 10.7554/eLife.59432. Elife. 2021. PMID: 34251337 Free PMC article.
-
Toward Understanding the Molecular Role of SNX27/Retromer in Human Health and Disease.Front Cell Dev Biol. 2021 Apr 15;9:642378. doi: 10.3389/fcell.2021.642378. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33937239 Free PMC article. Review.
-
Endosomal Sorting Protein SNX27 and Its Emerging Roles in Human Cancers.Cancers (Basel). 2022 Dec 22;15(1):70. doi: 10.3390/cancers15010070. Cancers (Basel). 2022. PMID: 36612066 Free PMC article. Review.
Cited by
-
A defect in the retromer accessory protein, SNX27, manifests by infantile myoclonic epilepsy and neurodegeneration.Neurogenetics. 2015 Jul;16(3):215-221. doi: 10.1007/s10048-015-0446-0. Epub 2015 Apr 17. Neurogenetics. 2015. PMID: 25894286 Free PMC article.
-
GPCR Signaling and Trafficking: The Long and Short of It.Trends Endocrinol Metab. 2017 Mar;28(3):213-226. doi: 10.1016/j.tem.2016.10.007. Epub 2016 Nov 23. Trends Endocrinol Metab. 2017. PMID: 27889227 Free PMC article. Review.
-
Sorting Nexin 27 Enables MTOC and Secretory Machinery Translocation to the Immune Synapse.Front Immunol. 2022 Jan 12;12:814570. doi: 10.3389/fimmu.2021.814570. eCollection 2021. Front Immunol. 2022. PMID: 35095913 Free PMC article.
-
Quinoline-Malononitrile-Based Aggregation-Induced Emission Probe for Monoamine Oxidase Detection in Living Cells.Molecules. 2023 Mar 15;28(6):2655. doi: 10.3390/molecules28062655. Molecules. 2023. PMID: 36985627 Free PMC article.
-
Structural and Functional Consequences of Connexin 36 (Cx36) Interaction with Calmodulin.Front Mol Neurosci. 2016 Nov 18;9:120. doi: 10.3389/fnmol.2016.00120. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27917108 Free PMC article.
References
-
- Zhu J. J., Qin Y., Zhao M., Van Aelst L. & Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell 110, 443–455 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

