ChREBP, a glucose-responsive transcriptional factor, enhances glucose metabolism to support biosynthesis in human cytomegalovirus-infected cells
- PMID: 24449882
- PMCID: PMC3918764
- DOI: 10.1073/pnas.1310779111
ChREBP, a glucose-responsive transcriptional factor, enhances glucose metabolism to support biosynthesis in human cytomegalovirus-infected cells
Abstract
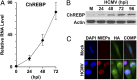
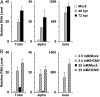
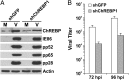
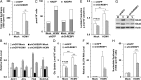
Carbohydrate-response element binding protein (ChREBP) plays a key role in regulating glucose metabolism and de novo lipogenesis in metabolic tissues and cancer cells. Here we report that ChREBP is also a critical regulator of the metabolic alterations induced during human cytomegalovirus (HCMV) infection. The expression of both ChREBP-α and ChREBP-β is robustly induced in HCMV-infected human fibroblasts; this induction is required for efficient HCMV infection. Depletion of ChREBP in HCMV-infected cells results in reduction of HCMV-induced glucose transporter 4 and glucose transporter 2 expression, leading to inhibition of glucose uptake, lactate production, nucleotide biosynthesis, and NADPH generation. We previously reported that HCMV infection induces lipogenesis through the activation of sterol regulatory element binding protein 1, which is mediated by the induction of PKR-like endoplasmic reticulum kinase. Data from the present study show that HCMV-induced lipogenesis is also controlled by the induction of ChREBP, in a second mechanism involved in the regulation of HCMV-induced de novo lipogenesis. These results suggest that ChREBP plays a key role in reprogramming glucose and lipid metabolism in HCMV infection.
Keywords: glycolysis; lipid synthesis; viral pathogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
PKR-like endoplasmic reticulum kinase is necessary for lipogenic activation during HCMV infection.PLoS Pathog. 2013;9(4):e1003266. doi: 10.1371/journal.ppat.1003266. Epub 2013 Apr 4. PLoS Pathog. 2013. PMID: 23592989 Free PMC article.
-
A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism.Nature. 2012 Apr 19;484(7394):333-8. doi: 10.1038/nature10986. Nature. 2012. PMID: 22466288 Free PMC article.
-
Identification of HNF-4α as a key transcription factor to promote ChREBP expression in response to glucose.Sci Rep. 2016 Mar 31;6:23944. doi: 10.1038/srep23944. Sci Rep. 2016. PMID: 27029511 Free PMC article.
-
ChREBP: a glucose-activated transcription factor involved in the development of metabolic syndrome.Endocr J. 2008 Aug;55(4):617-24. doi: 10.1507/endocrj.k07e-110. Epub 2008 May 19. Endocr J. 2008. PMID: 18490833 Review.
-
The Protective Role of the Carbohydrate Response Element Binding Protein in the Liver: The Metabolite Perspective.Front Endocrinol (Lausanne). 2020 Nov 17;11:594041. doi: 10.3389/fendo.2020.594041. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33281747 Free PMC article. Review.
Cited by
-
Adipocyte browning and resistance to obesity in mice is induced by expression of ATF3.Commun Biol. 2019 Oct 24;2:389. doi: 10.1038/s42003-019-0624-y. eCollection 2019. Commun Biol. 2019. PMID: 31667363 Free PMC article.
-
The Function of MondoA and ChREBP Nutrient-Sensing Factors in Metabolic Disease.Int J Mol Sci. 2023 May 16;24(10):8811. doi: 10.3390/ijms24108811. Int J Mol Sci. 2023. PMID: 37240157 Free PMC article. Review.
-
Melatonin Lowers HIF-1α Content in Human Proximal Tubular Cells (HK-2) Due to Preventing Its Deacetylation by Sirtuin 1.Front Physiol. 2021 Jan 14;11:572911. doi: 10.3389/fphys.2020.572911. eCollection 2020. Front Physiol. 2021. PMID: 33519498 Free PMC article.
-
Nocturnal oxygen therapy as an option for early COVID-19.Int J Infect Dis. 2020 Sep;98:176-179. doi: 10.1016/j.ijid.2020.06.080. Epub 2020 Jun 26. Int J Infect Dis. 2020. PMID: 32599285 Free PMC article.
-
The Role of Lipid Metabolism in COVID-19 Virus Infection and as a Drug Target.Int J Mol Sci. 2020 May 17;21(10):3544. doi: 10.3390/ijms21103544. Int J Mol Sci. 2020. PMID: 32429572 Free PMC article. Review.
References
-
- Pass RF, Fowler KB, Boppana SB, Britt WJ, Stagno S. Congenital cytomegalovirus infection following first trimester maternal infection: Symptoms at birth and outcome. J Clin Virol. 2006;35(2):216–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

