Immune responses to HCV and other hepatitis viruses
- PMID: 24439265
- PMCID: PMC4480226
- DOI: 10.1016/j.immuni.2013.12.010
Immune responses to HCV and other hepatitis viruses
Abstract
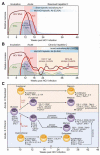
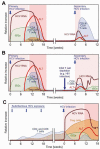
Five human hepatitis viruses cause most of the acute and chronic liver disease worldwide. Over the past 25 years, hepatitis C virus (HCV) in particular has received much interest because of its ability to persist in most immunocompetent adults and because of the lack of a protective vaccine. Here we examine innate and adaptive immune responses to HCV infection. Although HCV activates an innate immune response, it employs an elaborate set of mechanisms to evade interferon (IFN)-based antiviral immunity. By comparing innate and adaptive immune responses to HCV with those to hepatitis A and B viruses, we suggest that prolonged innate immune activation by HCV impairs the development of successful adaptive immune responses. Comparative immunology provides insights into the maintenance of immune protection. We conclude by discussing prospects for an HCV vaccine and future research needs for the hepatitis viruses.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Innate immune responses in hepatitis C virus infection.Semin Immunopathol. 2013 Jan;35(1):53-72. doi: 10.1007/s00281-012-0332-x. Epub 2012 Aug 7. Semin Immunopathol. 2013. PMID: 22868377 Free PMC article. Review.
-
Systematic identification of anti-interferon function on hepatitis C virus genome reveals p7 as an immune evasion protein.Proc Natl Acad Sci U S A. 2017 Feb 21;114(8):2018-2023. doi: 10.1073/pnas.1614623114. Epub 2017 Feb 3. Proc Natl Acad Sci U S A. 2017. PMID: 28159892 Free PMC article.
-
Innate immunity against hepatitis C virus.Curr Opin Immunol. 2016 Oct;42:98-104. doi: 10.1016/j.coi.2016.06.009. Epub 2016 Jun 28. Curr Opin Immunol. 2016. PMID: 27366996 Review.
-
Immune system control of hepatitis C virus infection.Curr Opin Virol. 2021 Feb;46:36-44. doi: 10.1016/j.coviro.2020.10.002. Epub 2020 Nov 1. Curr Opin Virol. 2021. PMID: 33137689 Free PMC article. Review.
-
Innate and adaptive immune responses in HCV infections.J Hepatol. 2014 Nov;61(1 Suppl):S14-25. doi: 10.1016/j.jhep.2014.06.035. Epub 2014 Nov 3. J Hepatol. 2014. PMID: 25443342 Review.
Cited by
-
Early interferon lambda production is induced by double-stranded RNA in iPS-derived hepatocyte-like cells.Oxf Open Immunol. 2024 Jun 6;5(1):iqae004. doi: 10.1093/oxfimm/iqae004. eCollection 2024. Oxf Open Immunol. 2024. PMID: 39193476 Free PMC article.
-
Hepatitis C Virus (HCV) Infection: Pathogenesis, Oral Manifestations, and the Role of Direct-Acting Antiviral Therapy: A Narrative Review.J Clin Med. 2024 Jul 9;13(14):4012. doi: 10.3390/jcm13144012. J Clin Med. 2024. PMID: 39064052 Free PMC article. Review.
-
Contemporary Insights into Hepatitis C Virus: A Comprehensive Review.Microorganisms. 2024 May 21;12(6):1035. doi: 10.3390/microorganisms12061035. Microorganisms. 2024. PMID: 38930417 Free PMC article. Review.
-
A Review on Role of Inflammation in Coronavirus Disease.Endocr Metab Immune Disord Drug Targets. 2024;24(13):1488-1505. doi: 10.2174/0118715303265274231204075802. Endocr Metab Immune Disord Drug Targets. 2024. PMID: 38303532 Review.
-
Therapeutic strategies and promising vaccine for hepatitis C virus infection.Immun Inflamm Dis. 2023 Aug;11(8):e977. doi: 10.1002/iid3.977. Immun Inflamm Dis. 2023. PMID: 37647422 Free PMC article. Review.
References
-
- Bellecave P, Sarasin-Filipowicz M, Donze O, Kennel A, Gouttenoire J, Meylan E, Terracciano L, Tschopp J, Sarrazin C, Berg T, et al. Cleavage of mitochondrial antiviral signaling protein in the liver of patients with chronic hepatitis C correlates with a reduced activation of the endogenous interferon system. Hepatology. 2010;51:1127–1136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

