Population pharmacokinetics of levodopa in subjects with advanced Parkinson's disease: levodopa-carbidopa intestinal gel infusion vs. oral tablets
- PMID: 24433449
- PMCID: PMC4168384
- DOI: 10.1111/bcp.12324
Population pharmacokinetics of levodopa in subjects with advanced Parkinson's disease: levodopa-carbidopa intestinal gel infusion vs. oral tablets
Abstract
Aims: Levodopa-carbidopa intestinal gel (LCIG) provides continuous levodopa-carbidopa delivery through intrajejunal infusion. This study characterized the population pharmacokinetics of levodopa following a 16 h jejunal infusion of LCIG or frequent oral administration of levodopa-carbidopa tablets (LC-oral) in subjects with advanced Parkinson's disease (PD).
Methods: A non-linear mixed-effects model of levodopa pharmacokinetics was developed using serial plasma concentrations from an LCIG phase 1 study and a phase 3 double-blind, double-dummy study of the efficacy and safety of LCIG compared with LC-oral in advanced PD patients (n = 68 for model development; 45 on LCIG and 23 on LC-oral). The final model was internally evaluated using stochastic simulations and bootstrap and externally evaluated using sparse pharmacokinetic data from 311 subjects treated in a long term safety study of LCIG.
Results: The final model was a two compartment model with a transit compartment for absorption, first order elimination, bioavailability for LCIG (97%; confidence interval = 95% to 98%) relative to LC-oral, different first order transit absorption rate constants (LCIG = 9.2 h(-1) vs. LC-oral = 2.4 h(-1) ; corresponding mean absorption time of 7 min for LCIG vs. 25 min for LC-oral) and different residual (intra-subject) variability for LCIG (15% proportional error, 0.3 μg ml(-1) additive error) vs. LC-oral (29% proportional error, 0.59 μg ml(-1) additive error). Estimated oral clearance and steady-state volume of distribution for levodopa were 24.8 l h(-1) and 131 l, respectively.
Conclusions: LCIG administration results in faster absorption, comparable levodopa bioavailability and significantly reduced intra-subject variability in levodopa concentrations relative to LC-oral administration.
Keywords: Duodopa; Parkinson's disease; intestinal gel; levodopa; population pharmacokinetics.
© 2014 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of The British Pharmacological Society.
Figures
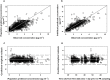
 , observed median;
, observed median;  , observed P5 and P95;
, observed P5 and P95;  , 95% CI for simulated median, P5 and P95
, 95% CI for simulated median, P5 and P95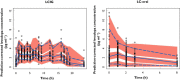
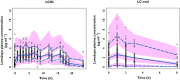
 , observed median;
, observed median;  , observed P5 and P95;
, observed P5 and P95;  , 95% CI for simulated median, P5 and P95
, 95% CI for simulated median, P5 and P95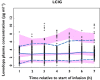
 , observed median;
, observed median;  , observed 5th and 95th percentiles;
, observed 5th and 95th percentiles;  , 95% CI for simulated median, 5th and 95th percentiles
, 95% CI for simulated median, 5th and 95th percentiles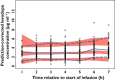
 , observed median;
, observed median;  , observed 5th and 95th percentiles;
, observed 5th and 95th percentiles;  , 95% CI for simulated median, 5th and 95th percentiles
, 95% CI for simulated median, 5th and 95th percentilesSimilar articles
-
Jejunal Infusion of levodopa-carbidopa intestinal gel versus oral administration of levodopa-carbidopa tablets in japanese subjects with advanced Parkinson's disease: pharmacokinetics and pilot efficacy and safety.Clin Pharmacokinet. 2015 Sep;54(9):975-84. doi: 10.1007/s40262-015-0265-3. Clin Pharmacokinet. 2015. PMID: 25875940 Free PMC article. Clinical Trial.
-
Levodopa-Carbidopa Intestinal Gel Pharmacokinetics: Lower Variability than Oral Levodopa-Carbidopa.J Parkinsons Dis. 2017;7(2):275-278. doi: 10.3233/JPD-161042. J Parkinsons Dis. 2017. PMID: 28211816 Free PMC article. Clinical Trial.
-
Pharmacokinetics of levodopa, carbidopa, and 3-O-methyldopa following 16-hour jejunal infusion of levodopa-carbidopa intestinal gel in advanced Parkinson's disease patients.AAPS J. 2013 Apr;15(2):316-23. doi: 10.1208/s12248-012-9439-1. Epub 2012 Dec 11. AAPS J. 2013. PMID: 23229334 Free PMC article.
-
Current Practices for Outpatient Initiation of Levodopa-Carbidopa Intestinal Gel for Management of Advanced Parkinson's Disease in the United States.Adv Ther. 2019 Sep;36(9):2233-2246. doi: 10.1007/s12325-019-01014-4. Epub 2019 Jul 5. Adv Ther. 2019. PMID: 31278691 Free PMC article. Review.
-
Levodopa-Carbidopa Intestinal Gel in Patients with Parkinson's Disease: A Systematic Review.CNS Drugs. 2016 May;30(5):381-404. doi: 10.1007/s40263-016-0336-5. CNS Drugs. 2016. PMID: 27138916 Review.
Cited by
-
Investigating Stochastic Differential Equations Modelling for Levodopa Infusion in Patients with Parkinson's Disease.Eur J Drug Metab Pharmacokinet. 2020 Feb;45(1):41-49. doi: 10.1007/s13318-019-00580-w. Eur J Drug Metab Pharmacokinet. 2020. PMID: 31595429 Free PMC article.
-
Intrajejunal Infusion of Levodopa-Carbidopa Gel Can Continuously Reduce the Severity of Dropped Head in Parkinson's Disease.Front Neurol. 2017 Oct 16;8:547. doi: 10.3389/fneur.2017.00547. eCollection 2017. Front Neurol. 2017. PMID: 29085331 Free PMC article.
-
Jejunal Infusion of levodopa-carbidopa intestinal gel versus oral administration of levodopa-carbidopa tablets in japanese subjects with advanced Parkinson's disease: pharmacokinetics and pilot efficacy and safety.Clin Pharmacokinet. 2015 Sep;54(9):975-84. doi: 10.1007/s40262-015-0265-3. Clin Pharmacokinet. 2015. PMID: 25875940 Free PMC article. Clinical Trial.
-
Population pharmacokinetics of levodopa/carbidopa microtablets in healthy subjects and Parkinson's disease patients.Eur J Clin Pharmacol. 2018 Oct;74(10):1299-1307. doi: 10.1007/s00228-018-2497-2. Epub 2018 Jun 7. Eur J Clin Pharmacol. 2018. PMID: 29882153 Free PMC article.
-
Effect of entacapone on colon motility and ion transport in a rat model of Parkinson's disease.World J Gastroenterol. 2015 Mar 28;21(12):3509-18. doi: 10.3748/wjg.v21.i12.3509. World J Gastroenterol. 2015. PMID: 25834315 Free PMC article.
References
-
- Nussbaum RL, Ellis CE. Alzheimer's disease and Parkinson's disease. N Engl J Med. 2003;348:1356–1364. - PubMed
-
- Hornykiewicz O. Basic research on dopamine in Parkinson's disease and the discovery of the nigrostriatal dopamine pathway: the view of an eyewitness. Neurodegener Dis. 2008;5:114–117. - PubMed
-
- Nutt JG. Pharmacokinetics and pharmacodynamics of levodopa. Mov Disord. 2008;23(Suppl. 3):S580–584. - PubMed
-
- Agid Y, Ahlskog E, Albanese A, Calne D, Chase T, De Yebenes J, Factor S, Fahn S, Gershanik O, Goetz C, Koller W, Kurth M, Lang A, Lees A, Lewitt P, Marsden D, Melamed E, Michel PP, Mizuno Y, Obeso J, Oertel W, Olanow W, Poewe W, Pollak P, Tolosa E. Levodopa in the treatment of Parkinson's disease: a consensus meeting. Mov Disord. 1999;14:911–913. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

