Pulmonary lymphangiectasia resulting from vascular endothelial growth factor-C overexpression during a critical period
- PMID: 24429550
- PMCID: PMC3969887
- DOI: 10.1161/CIRCRESAHA.114.303119
Pulmonary lymphangiectasia resulting from vascular endothelial growth factor-C overexpression during a critical period
Abstract
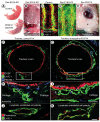
Rationale: Lymphatic vessels in the respiratory tract normally mature into a functional network during the neonatal period, but under some pathological conditions they can grow as enlarged, dilated sacs that result in the potentially lethal condition of pulmonary lymphangiectasia.
Objective: We sought to determine whether overexpression of the lymphangiogenic growth factor (vascular endothelial growth factor-C [VEGF-C]) can promote lymphatic growth and maturation in the respiratory tract. Unexpectedly, perinatal overexpression of VEGF-C in the respiratory epithelium led to a condition resembling human pulmonary lymphangiectasia, a life-threatening disorder of the newborn characterized by respiratory distress and the presence of widely dilated lymphatics.
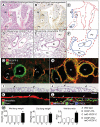
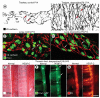
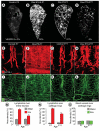
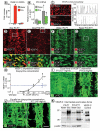
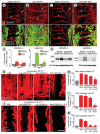
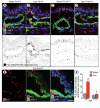
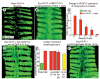
Methods and results: Administration of doxycycline to Clara cell secretory protein-reverse tetracycline-controlled transactivator/tetracycline operator-VEGF-C double-transgenic mice during a critical period from embryonic day 15.5 to postnatal day 14 was accompanied by respiratory distress, chylothorax, pulmonary lymphangiectasia, and high mortality. Enlarged sac-like lymphatics were abundant near major airways, pulmonary vessels, and visceral pleura. Side-by-side comparison revealed morphological features similar to pulmonary lymphangiectasia in humans. The condition was milder in mice given doxycycline after age postnatal day 14 and did not develop after postnatal day 35. Mechanistic studies revealed that VEGF recptor (VEGFR)-3 alone drove lymphatic growth in adult mice, but both VEGFR-2 and VEGFR-3 were required for the development of lymphangiectasia in neonates. VEGFR-2/VEGFR-3 heterodimers were more abundant in the dilated lymphatics, consistent with the involvement of both receptors. Despite the dependence of lymphangiectasia on VEGFR-2 and VEGFR-3, the condition was not reversed by blocking both receptors together or by withdrawing VEGF-C.
Conclusions: The findings indicate that VEGF-C overexpression can induce pulmonary lymphangiectasia during a critical period in perinatal development.
Keywords: VEGFR-2; VEGFR-3; chylothorax; lung; lymphangiogenesis; lymphangiomatosis, pulmonary; lymphatic vessels; pulmonary edema.
Figures
Similar articles
-
Differential Receptor Binding and Regulatory Mechanisms for the Lymphangiogenic Growth Factors Vascular Endothelial Growth Factor (VEGF)-C and -D.J Biol Chem. 2016 Dec 30;291(53):27265-27278. doi: 10.1074/jbc.M116.736801. Epub 2016 Nov 16. J Biol Chem. 2016. PMID: 27852824 Free PMC article.
-
Transgenic induction of vascular endothelial growth factor-C is strongly angiogenic in mouse embryos but leads to persistent lymphatic hyperplasia in adult tissues.Am J Pathol. 2008 Dec;173(6):1891-901. doi: 10.2353/ajpath.2008.080378. Epub 2008 Nov 6. Am J Pathol. 2008. PMID: 18988807 Free PMC article.
-
Hyperplasia, de novo lymphangiogenesis, and lymphatic regression in mice with tissue-specific, inducible overexpression of murine VEGF-D.Am J Physiol Heart Circ Physiol. 2016 Aug 1;311(2):H384-94. doi: 10.1152/ajpheart.00208.2016. Epub 2016 Jun 24. Am J Physiol Heart Circ Physiol. 2016. PMID: 27342876
-
Congenital Pulmonary Lymphangiectasia: A Disorder not only of Fetoneonates.Klin Padiatr. 2017 Jul;229(4):205-208. doi: 10.1055/s-0043-112500. Epub 2017 Jul 17. Klin Padiatr. 2017. PMID: 28718185 Review.
-
Molecular control of lymphatic metastasis.Ann N Y Acad Sci. 2008;1131:225-34. doi: 10.1196/annals.1413.020. Ann N Y Acad Sci. 2008. PMID: 18519975 Review.
Cited by
-
Vascular endothelial cell specification in health and disease.Angiogenesis. 2021 May;24(2):213-236. doi: 10.1007/s10456-021-09785-7. Epub 2021 Apr 12. Angiogenesis. 2021. PMID: 33844116 Free PMC article. Review.
-
Unexpected contribution of lymphatic vessels to promotion of distant metastatic tumor spread.Sci Adv. 2018 Aug 8;4(8):eaat4758. doi: 10.1126/sciadv.aat4758. eCollection 2018 Aug. Sci Adv. 2018. PMID: 30101193 Free PMC article.
-
Mechanisms and regulation of endothelial VEGF receptor signalling.Nat Rev Mol Cell Biol. 2016 Oct;17(10):611-25. doi: 10.1038/nrm.2016.87. Epub 2016 Jul 27. Nat Rev Mol Cell Biol. 2016. PMID: 27461391 Review.
-
VEGF-C promotes the development of lymphatics in bone and bone loss.Elife. 2018 Apr 5;7:e34323. doi: 10.7554/eLife.34323. Elife. 2018. PMID: 29620526 Free PMC article.
-
Modulation of Endothelial Bone Morphogenetic Protein Receptor Type 2 Activity by Vascular Endothelial Growth Factor Receptor 3 in Pulmonary Arterial Hypertension.Circulation. 2017 Jun 6;135(23):2288-2298. doi: 10.1161/CIRCULATIONAHA.116.025390. Epub 2017 Mar 29. Circulation. 2017. PMID: 28356442 Free PMC article.
References
-
- Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. - PubMed
-
- Bredt H. lymphangiectasia pulmonum congenita. Virchows Arch. 1952;321:517–530. - PubMed
-
- Laurence KM. Congenital pulmonary cystic lymphangiectasis. J Pathol Bacteriol. 1955;70:325–333. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

