Exploring Anopheles gut bacteria for Plasmodium blocking activity
- PMID: 24428613
- PMCID: PMC4099322
- DOI: 10.1111/1462-2920.12381
Exploring Anopheles gut bacteria for Plasmodium blocking activity
Abstract
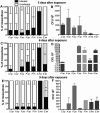
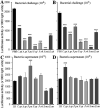
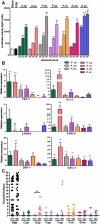
Malaria parasite transmission requires the successful development of Plasmodium gametocytes into flagellated microgametes upon mosquito blood ingestion, and the subsequent fertilization of microgametes and macrogametes for the development of motile zygotes, called ookinetes, which invade and transverse the Anopheles vector mosquito midgut at around 18-36 h after blood ingestion. Within the mosquito midgut, the malaria parasite has to withstand the mosquito's innate immune response and the detrimental effect of its commensal bacterial flora. We have assessed the midgut colonization capacity of five gut bacterial isolates from field-derived, and two from laboratory colony, mosquitoes and their effect on Plasmodium development in vivo and in vitro, along with their impact on mosquito survival. Some bacterial isolates activated the mosquito's immune system, affected the mosquito's lifespan, and were capable of blocking Plasmodium development. We have also shown that the ability of these bacteria to inhibit the parasites is likely to involve different mechanisms and factors. A Serratia marcescens isolate was particularly efficient in colonizing the mosquitoes' gut, compromising mosquito survival and inhibiting both Plasmodium sexual- and asexual-stage through secreted factors, thereby rendering it a potential candidate for the development of a malaria transmission intervention strategy.
© 2014 Society for Applied Microbiology and John Wiley & Sons Ltd.
Figures
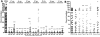

Similar articles
-
Intra-specific diversity of Serratia marcescens in Anopheles mosquito midgut defines Plasmodium transmission capacity.Sci Rep. 2013;3:1641. doi: 10.1038/srep01641. Sci Rep. 2013. PMID: 23571408 Free PMC article.
-
Mosquito ingestion of antibodies against mosquito midgut microbiota improves conversion of ookinetes to oocysts for Plasmodium falciparum, but not P. yoelii.Parasitol Int. 2011 Dec;60(4):440-6. doi: 10.1016/j.parint.2011.07.007. Epub 2011 Jul 13. Parasitol Int. 2011. PMID: 21763778 Free PMC article.
-
Caudal is a negative regulator of the Anopheles IMD pathway that controls resistance to Plasmodium falciparum infection.Dev Comp Immunol. 2013 Apr;39(4):323-32. doi: 10.1016/j.dci.2012.10.009. Epub 2012 Nov 22. Dev Comp Immunol. 2013. PMID: 23178401 Free PMC article.
-
The Anopheles innate immune system in the defense against malaria infection.J Innate Immun. 2014;6(2):169-81. doi: 10.1159/000353602. Epub 2013 Aug 28. J Innate Immun. 2014. PMID: 23988482 Free PMC article. Review.
-
Interactions of human malaria parasites, Plasmodium vivax and P.falciparum, with the midgut of Anopheles mosquitoes.Med Vet Entomol. 1997 Jul;11(3):290-6. doi: 10.1111/j.1365-2915.1997.tb00409.x. Med Vet Entomol. 1997. PMID: 9330262 Review.
Cited by
-
Interactions between West Nile Virus and the Microbiota of Culex pipiens Vectors: A Literature Review.Pathogens. 2023 Oct 27;12(11):1287. doi: 10.3390/pathogens12111287. Pathogens. 2023. PMID: 38003752 Free PMC article. Review.
-
Mosquito C-type lectins maintain gut microbiome homeostasis.Nat Microbiol. 2016 May;1:16023. doi: 10.1038/nmicrobiol.2016.23. Epub 2016 Mar 14. Nat Microbiol. 2016. PMID: 27170846 Free PMC article.
-
The mosquito microbiome includes habitat-specific but rare symbionts.Comput Struct Biotechnol J. 2021 Dec 23;20:410-420. doi: 10.1016/j.csbj.2021.12.019. eCollection 2022. Comput Struct Biotechnol J. 2021. PMID: 35140881 Free PMC article.
-
Reduced diversity of gut microbiota in two Aedes mosquitoes species in areas of recent invasion.Sci Rep. 2018 Oct 31;8(1):16091. doi: 10.1038/s41598-018-34640-z. Sci Rep. 2018. PMID: 30382151 Free PMC article.
-
Antimicrobial Resistance and Comparative Genomic Analysis of Elizabethkingia anophelis subsp. endophytica Isolated from Raw Milk.Antibiotics (Basel). 2022 May 12;11(5):648. doi: 10.3390/antibiotics11050648. Antibiotics (Basel). 2022. PMID: 35625292 Free PMC article.
References
-
- Castro DP, Seabra SH, Garcia ES, de Souza W, Azambuja P. Trypanosoma cruzi: ultrastructural studies of adhesion, lysis and biofilm formation by Serratia marcescens. Exp Parasitol. 2007;117:201–207. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

