Effect of TTP488 in patients with mild to moderate Alzheimer's disease
- PMID: 24423155
- PMCID: PMC4021072
- DOI: 10.1186/1471-2377-14-12
Effect of TTP488 in patients with mild to moderate Alzheimer's disease
Abstract
Background: TTP488, an antagonist at the Receptor for Advanced Glycation End products, was evaluated as a potential treatment for patients with mild-to-moderate Alzheimer's disease (AD). A previous report describes decreased decline in ADAS-cog (delta = 3.1, p = 0.008 at 18 months, ANCOVA with multiple imputation), relative to placebo, following a 5 mg/day dose of TTP488. Acute, reversible cognitive worsening was seen with a 20 mg/day dose. The present study further evaluates the efficacy of TTP488 by subgroup analyses based on disease severity and concentration effect analysis.
Methods: 399 patients were randomized to one of two oral TTP488 doses (60 mg for 6 days followed by 20 mg/day; 15 mg for 6 days followed by 5 mg/day) or placebo for 18 months. Pre-specified primary analysis, using an ITT population, was on the ADAS-cog11. Secondary analyses included as a key secondary variable the Clinical Dementia Rating-Sum of Boxes (CDR-SB), and another secondary variable of the ADCS-ADL.
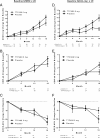
Results: On-treatment analysis demonstrated numerical differences favoring 5 mg/day over placebo, with nominal significance at Month 18 (delta = 2.7, p = 0.03). Patients with mild AD, whether defined by MMSE or ADAS-cog, demonstrated significant differences favoring 5 mg/day on ADAS-cog and trends on CDR-sb and ADCS-ADL at Month 18. TTP488 plasma concentrations of 7.6-16.8 ng/mL were associated with a decreased decline in ADAS-cog over time compared to placebo. Worsening on the ADAS-cog relative to placebo was evident at 46.8-167.0 ng/mL.
Conclusions: Results of these analyses support further investigation of 5 mg/day in future Phase 3 trials in patients with mild AD.
Figures
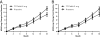

Similar articles
-
Efficacy and safety of tarenflurbil in mild to moderate Alzheimer's disease: a randomised phase II trial.Lancet Neurol. 2008 Jun;7(6):483-93. doi: 10.1016/S1474-4422(08)70090-5. Epub 2008 Apr 29. Lancet Neurol. 2008. PMID: 18450517 Clinical Trial.
-
Detecting Treatment Group Differences in Alzheimer's Disease Clinical Trials: A Comparison of Alzheimer's Disease Assessment Scale - Cognitive Subscale (ADAS-Cog) and the Clinical Dementia Rating - Sum of Boxes (CDR-SB).J Prev Alzheimers Dis. 2018;5(1):15-20. doi: 10.14283/jpad.2018.2. J Prev Alzheimers Dis. 2018. PMID: 29405227
-
Effect of tramiprosate in patients with mild-to-moderate Alzheimer's disease: exploratory analyses of the MRI sub-group of the Alphase study.J Nutr Health Aging. 2009 Jun;13(6):550-7. doi: 10.1007/s12603-009-0106-x. J Nutr Health Aging. 2009. PMID: 19536424 Clinical Trial.
-
Galantamine for Alzheimer's disease.Cochrane Database Syst Rev. 2001;(4):CD001747. doi: 10.1002/14651858.CD001747. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2002;(3):CD001747. doi: 10.1002/14651858.CD001747. PMID: 11687119 Updated. Review.
-
Galantamine for Alzheimer's disease.Cochrane Database Syst Rev. 2002;(3):CD001747. doi: 10.1002/14651858.CD001747. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2004 Oct 18;(4):CD001747. doi: 10.1002/14651858.CD001747.pub2. PMID: 12137632 Updated. Review.
Cited by
-
Soluble RAGE Treatment Delays Progression of Amyotrophic Lateral Sclerosis in SOD1 Mice.Front Cell Neurosci. 2016 May 9;10:117. doi: 10.3389/fncel.2016.00117. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27242430 Free PMC article.
-
Macrophage RAGE activation is proinflammatory in NASH.JCI Insight. 2024 Feb 8;9(3):e169138. doi: 10.1172/jci.insight.169138. JCI Insight. 2024. PMID: 38175729 Free PMC article.
-
RAGE signalling in obesity and diabetes: focus on the adipose tissue macrophage.Adipocyte. 2020 Dec;9(1):563-566. doi: 10.1080/21623945.2020.1817278. Adipocyte. 2020. PMID: 32892690 Free PMC article. Review.
-
Avenues for post-translational protein modification prevention and therapy.Mol Aspects Med. 2022 Aug;86:101083. doi: 10.1016/j.mam.2022.101083. Epub 2022 Feb 25. Mol Aspects Med. 2022. PMID: 35227517 Free PMC article. Review.
-
AGE-RAGE stress: a changing landscape in pathology and treatment of Alzheimer's disease.Mol Cell Biochem. 2019 Sep;459(1-2):95-112. doi: 10.1007/s11010-019-03553-4. Epub 2019 May 11. Mol Cell Biochem. 2019. PMID: 31079281 Review.
References
-
- Schmidt AM, Sahagan B, Nelson RB, Selmer J, Rothlein R, Bell JM. The role of RAGE in amyloid-beta peptide-mediated pathology in Alzheimer’s disease. Curr Opin Investig Drugs. 2009;10(7):672–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

