Novel regulation of CD80/CD86-induced phosphatidylinositol 3-kinase signaling by NOTCH1 protein in interleukin-6 and indoleamine 2,3-dioxygenase production by dendritic cells
- PMID: 24415757
- PMCID: PMC3953285
- DOI: 10.1074/jbc.M113.519686
Novel regulation of CD80/CD86-induced phosphatidylinositol 3-kinase signaling by NOTCH1 protein in interleukin-6 and indoleamine 2,3-dioxygenase production by dendritic cells
Abstract
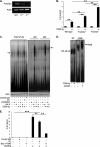
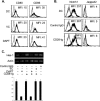
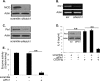
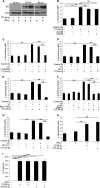
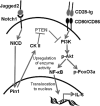
Dendritic cells (DC) play a critical role in modulating antigen-specific immune responses elicited by T cells via engagement of the prototypic T cell costimulatory receptor CD28 by the cognate ligands CD80/CD86, expressed on DC. Although CD28 signaling in T cell activation has been well characterized, it has only recently been shown that CD80/CD86, which have no demonstrated binding domains for signaling proteins in their cytoplasmic tails, nonetheless also transduce signals to the DC. Functionally, CD80/CD86 engagement results in DC production of the pro-inflammatory cytokine IL-6, which is necessary for full T cell activation. However, ligation of CD80/CD86 by CTLA4 also induces DC production of the immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO), which depletes local pools of the essential amino acid tryptophan, resulting in blockade of T cell activation. Despite the significant role of CD80/CD86 in immunological processes and the seemingly opposing roles they play by producing IL-6 and IDO upon their activation, how CD80/CD86 signal remains poorly understood. We have now found that cross-linking CD80/CD86 in human DC activates the PI3K/AKT pathway. This results in phosphorylation/inactivation of its downstream target, FOXO3A, and alleviates FOXO3A-mediated suppression of IL-6 expression. A second event downstream of AKT phosphorylation is activation of the canonical NF-κB pathway, which induces IL-6 expression. In addition to these downstream pathways, we unexpectedly found that CD80/CD86-induced PI3K signaling is regulated by previously unrecognized cross-talk with NOTCH1 signaling. This cross-talk is facilitated by NOTCH-mediated up-regulation of the expression of prolyl isomerase PIN1, which in turn increases enzyme activity of casein kinase II. Subsequently, phosphatase and tensin homolog (which suppresses PI3K activity) is inactivated via phosphorylation by casein kinase II. This results in full activation of PI3K signaling upon cross-linking CD80/CD86. Similar to IL-6, we have found that CD80/CD86-induced IDO production by DC at late time points is also dependent upon the PI3K → AKT → NF-κB pathway and requires cross-talk with NOTCH signaling. These data further suggest that the same signaling pathways downstream of DC CD80/CD86 cross-linking induce early IL-6 production to enhance T cell activation, followed by later IDO production to self-limit this activation. In addition to characterizing the pathways downstream of CD80/CD86 in IL-6 and IDO production, identification of a novel cross-talk between NOTCH1 and PI3K signaling may provide new insights in other biological processes where PI3K signaling plays a major role.
Keywords: Dendritic Cells; Indoleamine 2,3-Dioxygenase; Interleukin; NOTCH Pathway; PI 3-Kinase (PI3K); Signal Transduction.
Figures




Similar articles
-
SOCS3 drives proteasomal degradation of indoleamine 2,3-dioxygenase (IDO) and antagonizes IDO-dependent tolerogenesis.Proc Natl Acad Sci U S A. 2008 Dec 30;105(52):20828-33. doi: 10.1073/pnas.0810278105. Epub 2008 Dec 16. Proc Natl Acad Sci U S A. 2008. PMID: 19088199 Free PMC article.
-
Regulating the expression of CD80/CD86 on dendritic cells to induce immune tolerance after xeno-islet transplantation.Immunobiology. 2016 Jul;221(7):803-12. doi: 10.1016/j.imbio.2016.02.002. Epub 2016 Feb 3. Immunobiology. 2016. PMID: 26879762
-
Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells.J Immunol. 2004 Apr 1;172(7):4100-10. doi: 10.4049/jimmunol.172.7.4100. J Immunol. 2004. PMID: 15034022
-
New insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling axis: targeted therapy of γ-secretase inhibitor resistant T-cell acute lymphoblastic leukemia.Cell Signal. 2014 Jan;26(1):149-61. doi: 10.1016/j.cellsig.2013.09.021. Epub 2013 Oct 16. Cell Signal. 2014. PMID: 24140475 Review.
-
From Melanoma Development to RNA-Modified Dendritic Cell Vaccines: Highlighting the Lessons From the Past.Front Immunol. 2021 Feb 22;12:623639. doi: 10.3389/fimmu.2021.623639. eCollection 2021. Front Immunol. 2021. PMID: 33692796 Free PMC article. Review.
Cited by
-
Targeting Pin1 for Modulation of Cell Motility and Cancer Therapy.Biomedicines. 2021 Mar 31;9(4):359. doi: 10.3390/biomedicines9040359. Biomedicines. 2021. PMID: 33807199 Free PMC article. Review.
-
Changes in the BTK/NF-κB signaling pathway and related cytokines in different stages of neuromyelitis optica spectrum disorders.Eur J Med Res. 2022 Jun 21;27(1):96. doi: 10.1186/s40001-022-00723-x. Eur J Med Res. 2022. PMID: 35729649 Free PMC article.
-
Mycobacteria-responsive sonic hedgehog signaling mediates programmed death-ligand 1- and prostaglandin E2-induced regulatory T cell expansion.Sci Rep. 2016 Apr 15;6:24193. doi: 10.1038/srep24193. Sci Rep. 2016. PMID: 27080341 Free PMC article.
-
Stiffness regulates dendritic cell and macrophage subtype development and increased stiffness induces a tumor-associated macrophage phenotype in cancer co-cultures.Front Immunol. 2024 Aug 15;15:1434030. doi: 10.3389/fimmu.2024.1434030. eCollection 2024. Front Immunol. 2024. PMID: 39211033 Free PMC article.
-
Targeting Multiple Myeloma through the Biology of Long-Lived Plasma Cells.Cancers (Basel). 2020 Jul 30;12(8):2117. doi: 10.3390/cancers12082117. Cancers (Basel). 2020. PMID: 32751699 Free PMC article. Review.
References
-
- Lanier L. L., O'Fallon S., Somoza C., Phillips J. H., Linsley P. S., Okumura K., Ito D., Azuma M. (1995) CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J. Immunol. 154, 97–105 - PubMed
-
- Sharpe A. H., Freeman G. J. (2002) The B7-CD28 superfamily. Nat. Rev. Immunol. 2, 116–126 - PubMed
-
- Frauwirth K. A., Riley J. L., Harris M. H., Parry R. V., Rathmell J. C., Plas D. R., Elstrom R. L., June C. H., Thompson C. B. (2002) The CD28 signaling pathway regulates glucose metabolism. Immunity 16, 769–777 - PubMed
-
- Bour-Jordan H., Blueston J. A. (2002) CD28 function: a balance of costimulatory and regulatory signals. J. Clin. Immunol. 22, 1–7 - PubMed
-
- Orabona C., Grohmann U., Belladonna M. L., Fallarino F., Vacca C., Bianchi R., Bozza S., Volpi C., Salomon B. L., Fioretti M. C., Romani L., Puccetti P. (2004) CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat. Immunol. 5, 1134–1142 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

