Vortex-aided inertial microfluidic device for continuous particle separation with high size-selectivity, efficiency, and purity
- PMID: 24404052
- PMCID: PMC3765293
- DOI: 10.1063/1.4818906
Vortex-aided inertial microfluidic device for continuous particle separation with high size-selectivity, efficiency, and purity
Abstract
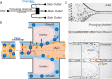
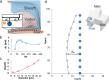
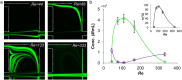
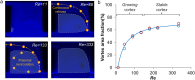
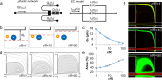
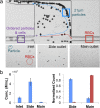
In this paper, we report an inertial microfluidic device with simple geometry for continuous extraction of large particles with high size-selectivity (<2 μm), high efficiency (∼90%), and high purity (>90%). The design takes advantage of a high-aspect-ratio microchannel to inertially equilibrate cells and symmetric chambers for microvortex-aided cell extraction. A side outlet in each chamber continuously siphons larger particles, while the smaller particles or cells exit through the main outlet. The design has several advantages, including simple design, small footprint, ease of paralleling and cascading, one-step operation, and continuous separation with ultra-selectivity, high efficiency and purity. The described approach is applied to manipulating cells and particles for ultra-selective separation, quickly and effectively extracting larger sizes from the main flow, with broad applications in cell separations.
Figures

Similar articles
-
Improvement of size-based particle separation throughput in slanted spiral microchannel by modifying outlet geometry.Electrophoresis. 2020 Mar;41(5-6):353-359. doi: 10.1002/elps.201900436. Epub 2020 Feb 13. Electrophoresis. 2020. PMID: 32012295
-
Inertial microfluidics for continuous particle separation in spiral microchannels.Lab Chip. 2009 Oct 21;9(20):2973-80. doi: 10.1039/b908271a. Epub 2009 Jul 21. Lab Chip. 2009. PMID: 19789752
-
A passive microfluidic device for continuous microparticle enrichment.Electrophoresis. 2019 Mar;40(6):1000-1009. doi: 10.1002/elps.201800454. Epub 2018 Dec 6. Electrophoresis. 2019. PMID: 30488639
-
A Review of Secondary Flow in Inertial Microfluidics.Micromachines (Basel). 2020 Apr 28;11(5):461. doi: 10.3390/mi11050461. Micromachines (Basel). 2020. PMID: 32354106 Free PMC article. Review.
-
Sorting of Particles Using Inertial Focusing and Laminar Vortex Technology: A Review.Micromachines (Basel). 2019 Sep 10;10(9):594. doi: 10.3390/mi10090594. Micromachines (Basel). 2019. PMID: 31510006 Free PMC article. Review.
Cited by
-
Single stream inertial focusing in a straight microchannel.Lab Chip. 2015 Apr 21;15(8):1812-21. doi: 10.1039/c4lc01462f. Lab Chip. 2015. PMID: 25761900 Free PMC article.
-
Elasto-Inertial Focusing Mechanisms of Particles in Shear-Thinning Viscoelastic Fluid in Rectangular Microchannels.Micromachines (Basel). 2022 Dec 1;13(12):2131. doi: 10.3390/mi13122131. Micromachines (Basel). 2022. PMID: 36557430 Free PMC article.
-
Virtual vortex gear: Unique flow patterns driven by microfluidic inertia leading to pinpoint injection.Biomicrofluidics. 2018 Jun 20;12(3):034114. doi: 10.1063/1.5031082. eCollection 2018 May. Biomicrofluidics. 2018. PMID: 29983839 Free PMC article.
-
Rapid isolation of blood plasma using a cascaded inertial microfluidic device.Biomicrofluidics. 2017 Mar 24;11(2):024109. doi: 10.1063/1.4979198. eCollection 2017 Mar. Biomicrofluidics. 2017. PMID: 28405258 Free PMC article.
-
A bioinspired, passive microfluidic lobe filtration system.Lab Chip. 2021 Sep 28;21(19):3762-3774. doi: 10.1039/d1lc00449b. Lab Chip. 2021. PMID: 34581374 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
