CXCR4 drives the metastatic phenotype in breast cancer through induction of CXCR2 and activation of MEK and PI3K pathways
- PMID: 24403602
- PMCID: PMC3937084
- DOI: 10.1091/mbc.E13-07-0360
CXCR4 drives the metastatic phenotype in breast cancer through induction of CXCR2 and activation of MEK and PI3K pathways
Abstract
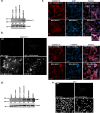
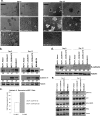
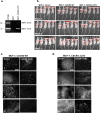
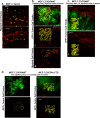
Aberrant expression of CXCR4 in human breast cancer correlates with metastasis to tissues secreting CXCL12. To understand the mechanism by which CXCR4 mediates breast cancer metastasis, MCF-7 breast carcinoma cells were transduced to express wild-type CXCR4 (CXCR4WT) or constitutively active CXCR4 (CXCR4ΔCTD) and analyzed in two-dimensional (2D) cultures, three-dimensional reconstituted basement membrane (3D rBM) cultures, and mice using intravital imaging. Two-dimensional cultures of MCF-7 CXCR4ΔCTD cells, but not CXCR4WT, exhibited an epithelial-to-mesenchymal transition (EMT) characterized by up-regulation of zinc finger E box-binding homeobox 1, loss of E-cadherin, up-regulation of cadherin 11, p120 isoform switching, activation of extracellular signal-regulated kinase 1/2, and matrix metalloproteinase-2. In contrast to the 2D environment, MCF-7 CXCR4WT cells cultured in 3D rBM exhibited an EMT phenotype, accompanied by expression of CXCR2, CXCR7, CXCL1, CXCL8, CCL2, interleukin-6, and granulocyte-macrophage colony stimulating factor. Dual inhibition of CXCR2 with CXCR4, or inhibition of either receptor with inhibitors of mitogen-activated protein kinase 1 or phosphatidylinositol 3-kinase, reversed the aggressive phenotype of MCF-7 CXCR4-expressing or MDA-MB-231 cells in 3D rBM. Intravital imaging of CXCR4-expressing MCF-7 cells revealed that tumor cells migrate toward blood vessels and metastasize to lymph nodes. Thus CXCR4 can drive EMT along with an up-regulation of chemokine receptors and cytokines important in cell migration, lymphatic invasion, and tumor metastasis.
Figures



Similar articles
-
miR-381 inhibited breast cancer cells proliferation, epithelial-to-mesenchymal transition and metastasis by targeting CXCR4.Biomed Pharmacother. 2017 Feb;86:426-433. doi: 10.1016/j.biopha.2016.12.051. Epub 2016 Dec 21. Biomed Pharmacother. 2017. PMID: 28012397
-
The N-terminal polypeptide derived from viral macrophage inflammatory protein II reverses breast cancer epithelial-to-mesenchymal transition via a PDGFRα-dependent mechanism.Oncotarget. 2017 Jun 6;8(23):37448-37463. doi: 10.18632/oncotarget.16394. Oncotarget. 2017. PMID: 28415580 Free PMC article.
-
Embelin Inhibits Invasion and Migration of MDA-MB-231 Breast Cancer Cells by Suppression of CXC Chemokine Receptor 4, Matrix Metalloproteinases-9/2, and Epithelial-Mesenchymal Transition.Phytother Res. 2016 Jun;30(6):1021-32. doi: 10.1002/ptr.5612. Epub 2016 Mar 31. Phytother Res. 2016. PMID: 27030214
-
Genes involved in breast cancer metastasis to bone.Cell Mol Life Sci. 2002 Sep;59(9):1491-502. doi: 10.1007/s00018-002-8524-5. Cell Mol Life Sci. 2002. PMID: 12440771 Free PMC article. Review.
-
Breast Cancer Metastasis: Mechanisms and Therapeutic Implications.Int J Mol Sci. 2022 Jun 18;23(12):6806. doi: 10.3390/ijms23126806. Int J Mol Sci. 2022. PMID: 35743249 Free PMC article. Review.
Cited by
-
Integrin alpha 10, CD44, PTEN, cadherin-11 and lactoferrin expressions are potential biomarkers for selecting patients in need of central nervous system prophylaxis in diffuse large B-cell lymphoma.Carcinogenesis. 2017 Aug 1;38(8):812-820. doi: 10.1093/carcin/bgx061. Carcinogenesis. 2017. PMID: 28854563 Free PMC article.
-
Application of a small molecule inhibitor screen approach to identify CXCR4 downstream signaling pathways that promote a mesenchymal and fulvestrant-resistant phenotype in breast cancer cells.Oncol Lett. 2021 May;21(5):380. doi: 10.3892/ol.2021.12641. Epub 2021 Mar 16. Oncol Lett. 2021. PMID: 33777204 Free PMC article.
-
Short-term cellular memory tunes the signaling responses of the chemokine receptor CXCR4.Sci Signal. 2019 Jul 9;12(589):eaaw4204. doi: 10.1126/scisignal.aaw4204. Sci Signal. 2019. PMID: 31289212 Free PMC article.
-
Wnt5a promotes migration of human osteosarcoma cells by triggering a phosphatidylinositol-3 kinase/Akt signals.Cancer Cell Int. 2014 Feb 14;14(1):15. doi: 10.1186/1475-2867-14-15. Cancer Cell Int. 2014. PMID: 24524196 Free PMC article.
-
Phosphorylation-Dependent Differences in CXCR4-LASP1-AKT1 Interaction between Breast Cancer and Chronic Myeloid Leukemia.Cells. 2020 Feb 14;9(2):444. doi: 10.3390/cells9020444. Cells. 2020. PMID: 32075106 Free PMC article.
References
-
- Ali S, O'Boyle G, Mellor P, Kirby JA. An apparent paradox: chemokine receptor agonists can be used for anti-inflammatory therapy. Mol Immunol. 2007;44:1477–1482. - PubMed
-
- Arihiro K, Oda H, Kaneko M, Inai K. Cytokines facilitate chemotactic motility of breast carcinoma cells. Breast Cancer. 2000;7:221–230. - PubMed
-
- Asgeirsson KS, Olafsdottir K, Jonasson JG, Ogmundsdottir HM. The effects of IL-6 on cell adhesion and e-cadherin expression in breast cancer. Cytokine. 1998;10:720–728. - PubMed
-
- Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005a;280:35760–35766. - PubMed
-
- Balabanian K, et al. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005b;105:2449–2457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

