Suppression of interferon lambda signaling by SOCS-1 results in their excessive production during influenza virus infection
- PMID: 24391501
- PMCID: PMC3879354
- DOI: 10.1371/journal.ppat.1003845
Suppression of interferon lambda signaling by SOCS-1 results in their excessive production during influenza virus infection
Erratum in
-
Correction: Suppression of Interferon Lambda Signaling by SOCS-1 Results in Their Excessive Production during Influenza Virus Infection.PLoS Pathog. 2016 Jan 14;12(1):e1005402. doi: 10.1371/journal.ppat.1005402. eCollection 2016 Jan. PLoS Pathog. 2016. PMID: 26765537 Free PMC article.
Abstract
Innate cytokine response provides the first line of defense against influenza virus infection. However, excessive production of cytokines appears to be critical in the pathogenesis of influenza virus. Interferon lambdas (IFN-λ) have been shown to be overproduced during influenza virus infection, but the precise pathogenic processes of IFN-λ production have yet to be characterized. In this report, we observed that influenza virus induced robust expression of IFN-λ in alveolar epithelial cells (A549) mainly through a RIG-I-dependent pathway, but IFN-λ-induced phosphorylation of the signal transducer and activator of transcription protein 1 (STAT1) was dramatically inhibited in the infected cells. Remarkably, influenza virus infection induced robust expression of suppressor of cytokine signaling-1 (SOCS-1), leading to inhibition of STAT1 activation. Interestingly, the virus-induced SOCS-1 expression was cytokine-independent at early stage of infection both in vitro and in vivo. Using transgenic mouse model and distinct approaches altering the expression of SOCS-1 or activation of STAT signaling, we demonstrated that disruption of the SOCS-1 expression or expression of constitutively active STAT1 significantly reduced the production of IFN-λ during influenza virus infection. Furthermore, we revealed that disruption of IFN-λ signaling pathway by increased SOCS-1 protein resulted in the activation of NF-κB and thereby enhanced the IFN-λ expression. Together, these data imply that suppression of IFN-λ signaling by virus-induced SOCS-1 causes an adaptive increase in IFN-λ expression by host to protect cells against the viral infection, as a consequence, leading to excessive production of IFN-λ with impaired antiviral response.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
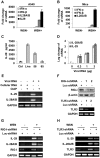
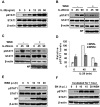
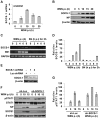
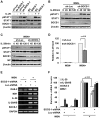
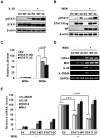
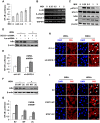

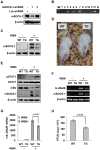
Similar articles
-
Influenza A virus inhibits type I IFN signaling via NF-kappaB-dependent induction of SOCS-3 expression.PLoS Pathog. 2008 Nov;4(11):e1000196. doi: 10.1371/journal.ppat.1000196. Epub 2008 Nov 7. PLoS Pathog. 2008. PMID: 18989459 Free PMC article.
-
IL-17A Attenuates IFN-λ Expression by Inducing Suppressor of Cytokine Signaling Expression in Airway Epithelium.J Immunol. 2018 Oct 15;201(8):2392-2402. doi: 10.4049/jimmunol.1800147. Epub 2018 Sep 17. J Immunol. 2018. PMID: 30224514
-
SOCS-1 inhibits expression of the antiviral proteins 2',5'-OAS and MxA induced by the novel interferon-lambdas IL-28A and IL-29.Biochem Biophys Res Commun. 2005 Jun 3;331(2):543-8. doi: 10.1016/j.bbrc.2005.04.004. Biochem Biophys Res Commun. 2005. PMID: 15850793
-
SOCS, Intrinsic Virulence Factors, and Treatment of COVID-19.Front Immunol. 2020 Oct 23;11:582102. doi: 10.3389/fimmu.2020.582102. eCollection 2020. Front Immunol. 2020. PMID: 33193390 Free PMC article. Review.
-
Interferon Lambda in the Pathogenesis of Inflammatory Bowel Diseases.Front Immunol. 2021 Oct 12;12:767505. doi: 10.3389/fimmu.2021.767505. eCollection 2021. Front Immunol. 2021. PMID: 34712246 Free PMC article. Review.
Cited by
-
Targeted disruption of influenza A virus hemagglutinin in genetically modified mice reduces viral replication and improves disease outcome.Sci Rep. 2016 Apr 1;6:23746. doi: 10.1038/srep23746. Sci Rep. 2016. PMID: 27033724 Free PMC article.
-
Cytokines and microRNAs in SARS-CoV-2: What do we know?Mol Ther Nucleic Acids. 2022 Sep 13;29:219-242. doi: 10.1016/j.omtn.2022.06.017. Epub 2022 Jun 25. Mol Ther Nucleic Acids. 2022. PMID: 35782361 Free PMC article. Review.
-
Impact of Obesity on Influenza A Virus Pathogenesis, Immune Response, and Evolution.Front Immunol. 2019 May 10;10:1071. doi: 10.3389/fimmu.2019.01071. eCollection 2019. Front Immunol. 2019. PMID: 31134099 Free PMC article. Review.
-
Infection with Classical Swine Fever Virus Induces Expression of Type III Interferons and Activates Innate Immune Signaling.Front Microbiol. 2017 Dec 19;8:2558. doi: 10.3389/fmicb.2017.02558. eCollection 2017. Front Microbiol. 2017. PMID: 29312239 Free PMC article.
-
Host-Pathogen Interactions in Gram-Positive Bacterial Pneumonia.Clin Microbiol Rev. 2019 May 29;32(3):e00107-18. doi: 10.1128/CMR.00107-18. Print 2019 Jun 19. Clin Microbiol Rev. 2019. PMID: 31142498 Free PMC article. Review.
References
-
- Kumagai Y, Takeuchi O, Kato H, Kumar H, Matsui K, et al. (2007) Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity 27: 240–252. - PubMed
-
- Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, et al. (2007) Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 445: 319–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

