The transcriptional regulator BZR1 mediates trade-off between plant innate immunity and growth
- PMID: 24381244
- PMCID: PMC3875382
- DOI: 10.7554/eLife.00983
The transcriptional regulator BZR1 mediates trade-off between plant innate immunity and growth
Abstract
The molecular mechanisms underlying the trade-off between plant innate immunity and steroid-mediated growth are controversial. Here, we report that activation of the transcription factor BZR1 is required and sufficient for suppression of immune signaling by brassinosteroids (BR). BZR1 induces the expression of several WRKY transcription factors that negatively control early immune responses. In addition, BZR1 associates with WRKY40 to mediate the antagonism between BR and immune signaling. We reveal that BZR1-mediated inhibition of immunity is particularly relevant when plant fast growth is required, such as during etiolation. Thus, BZR1 acts as an important regulator mediating the trade-off between growth and immunity upon integration of environmental cues. DOI: http://dx.doi.org/10.7554/eLife.00983.001.
Keywords: PAMPs; brassinosteroids; growth; innate immunity; transcription factors.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
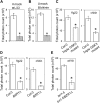
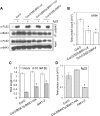
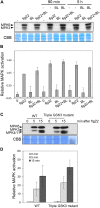
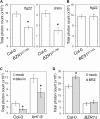
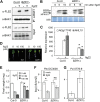

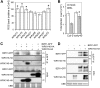
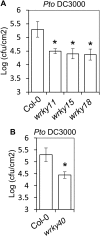
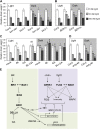
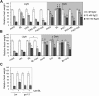

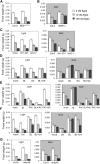
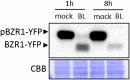
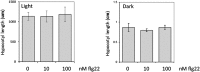
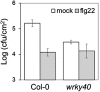
Similar articles
-
Trade-off between growth and immunity: role of brassinosteroids.Trends Plant Sci. 2015 Jan;20(1):12-9. doi: 10.1016/j.tplants.2014.09.003. Epub 2014 Sep 30. Trends Plant Sci. 2015. PMID: 25278266 Review.
-
The BZR1-EDS1 module regulates plant growth-defense coordination.Mol Plant. 2021 Dec 6;14(12):2072-2087. doi: 10.1016/j.molp.2021.08.011. Epub 2021 Aug 18. Mol Plant. 2021. PMID: 34416351
-
SUMO Conjugation to BZR1 Enables Brassinosteroid Signaling to Integrate Environmental Cues to Shape Plant Growth.Curr Biol. 2020 Apr 20;30(8):1410-1423.e3. doi: 10.1016/j.cub.2020.01.089. Epub 2020 Feb 27. Curr Biol. 2020. PMID: 32109396 Free PMC article.
-
An interaction between BZR1 and DELLAs mediates direct signaling crosstalk between brassinosteroids and gibberellins in Arabidopsis.Sci Signal. 2012 Oct 2;5(244):ra72. doi: 10.1126/scisignal.2002908. Sci Signal. 2012. PMID: 23033541
-
Molecular Mechanisms of Brassinosteroid-Mediated Responses to Changing Environments in Arabidopsis.Int J Mol Sci. 2020 Apr 15;21(8):2737. doi: 10.3390/ijms21082737. Int J Mol Sci. 2020. PMID: 32326491 Free PMC article. Review.
Cited by
-
Understanding the molecular mechanisms of trade-offs between plant growth and immunity.Sci China Life Sci. 2021 Feb;64(2):234-241. doi: 10.1007/s11427-020-1719-y. Epub 2020 Jul 17. Sci China Life Sci. 2021. PMID: 32710363 Review.
-
Effect of lipo-chitooligosaccharide on early growth of C4 grass seedlings.J Exp Bot. 2015 Sep;66(19):5727-38. doi: 10.1093/jxb/erv260. Epub 2015 Jun 6. J Exp Bot. 2015. PMID: 26049159 Free PMC article.
-
Genome-wide transcriptional profiling for elucidating the effects of brassinosteroids on Glycine max during early vegetative development.Sci Rep. 2019 Nov 6;9(1):16085. doi: 10.1038/s41598-019-52599-3. Sci Rep. 2019. PMID: 31695113 Free PMC article.
-
Abiotic Stresses Antagonize the Rice Defence Pathway through the Tyrosine-Dephosphorylation of OsMPK6.PLoS Pathog. 2015 Oct 20;11(10):e1005231. doi: 10.1371/journal.ppat.1005231. eCollection 2015 Oct. PLoS Pathog. 2015. PMID: 26485146 Free PMC article.
-
Flg22-Triggered Immunity Negatively Regulates Key BR Biosynthetic Genes.Front Plant Sci. 2015 Nov 9;6:981. doi: 10.3389/fpls.2015.00981. eCollection 2015. Front Plant Sci. 2015. PMID: 26617621 Free PMC article.
References
-
- Albrecht C, Boutrot F, Segonzac C, Schwessinger B, Gimenez-Ibanez S, Chinchilla D, Rathjen JP, de Vries SC, Zipfel C. 2012. Brassinosteroids inhibit pathogen-associated molecular pattern-triggered immune signaling independent of the receptor kinase BAK1. Proceedings of the National Academy of Sciences of the United States of America 109:303–308.10.1073/pnas.1109921108 - DOI - PMC - PubMed
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, Gadrinab C, Heller C, Jeske A, Koesema E, Meyers CC, Parker H, Prednis L, Ansari Y, Choy N, Deen H, Geralt M, Hazari N, Hom E, Karnes M, Mulholland C, Ndubaku R, Schmidt I, Guzman P, Aguilar-Henonin L, Schmid M, Weigel D, Carter DE, Marchand T, Risseeuw E, Brogden D, Zeko A, Crosby WL, Berry CC, Ecker JR. 2003. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301:653–657.10.1126/science.1086391 - DOI - PubMed
-
- Belkhadir Y, Jaillais Y, Epple P, Balsemao-Pires E, Dangl JL, Chory J. 2012. Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. Proceedings of the National Academy of Sciences of the United States of America 109:297–302.10.1073/pnas.1112840108 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

