Structural insight into the assembly of TRPV channels
- PMID: 24373766
- PMCID: PMC5548120
- DOI: 10.1016/j.str.2013.11.008
Structural insight into the assembly of TRPV channels
Abstract
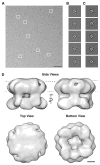
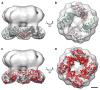
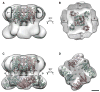
Transient receptor potential (TRP) proteins are a large family of polymodal nonselective cation channels. The TRP vanilloid (TRPV) subfamily consists of six homologous members with diverse functions. TRPV1-TRPV4 are nonselective cation channels proposed to play a role in nociception, while TRPV5 and TRPV6 are involved in epithelial Ca²⁺ homeostasis. Here we present the cryo-electron microscopy (cryo-EM) structure of functional, full-length TRPV2 at 13.6 Å resolution. The map reveals that the TRPV2 cytoplasmic domain displays a 4-fold petal-like shape in which high-resolution N-terminal ankyrin repeat domain (ARD) structures can be unambiguously fitted. Fitting of the available ARD structures for other TRPV subfamily members into the TRPV2 EM map suggests that TRPV subfamily members have highly homologous structural topologies. These results allowed us to postulate a structural explanation for the functional diversity among TRPV channels and their differential regulation by proteins and ligands.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Determining the Crystal Structure of TRPV6.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. PMID: 30299652 Free Books & Documents. Review.
-
Structural analyses of the ankyrin repeat domain of TRPV6 and related TRPV ion channels.Biochemistry. 2008 Feb 26;47(8):2476-84. doi: 10.1021/bi702109w. Epub 2008 Jan 31. Biochemistry. 2008. PMID: 18232717 Free PMC article.
-
Structure of the full-length TRPV2 channel by cryo-EM.Nat Commun. 2016 Mar 29;7:11130. doi: 10.1038/ncomms11130. Nat Commun. 2016. PMID: 27021073 Free PMC article.
-
Cryo-electron microscopy structure of the TRPV2 ion channel.Nat Struct Mol Biol. 2016 Feb;23(2):180-186. doi: 10.1038/nsmb.3159. Epub 2016 Jan 18. Nat Struct Mol Biol. 2016. PMID: 26779611 Free PMC article.
-
Pharmacology of vanilloid transient receptor potential cation channels.Mol Pharmacol. 2009 Jun;75(6):1262-79. doi: 10.1124/mol.109.055624. Epub 2009 Mar 18. Mol Pharmacol. 2009. PMID: 19297520 Review.
Cited by
-
Molecular details of ruthenium red pore block in TRPV channels.EMBO Rep. 2024 Feb;25(2):506-523. doi: 10.1038/s44319-023-00050-0. Epub 2024 Jan 15. EMBO Rep. 2024. PMID: 38225355 Free PMC article.
-
Potential therapeutic value of transient receptor potential channels in male urogenital system.Pflugers Arch. 2018 Nov;470(11):1583-1596. doi: 10.1007/s00424-018-2188-y. Epub 2018 Sep 7. Pflugers Arch. 2018. PMID: 30194638 Review.
-
Polymodal TRPV1 and TRPV4 Sensors Colocalize but Do Not Functionally Interact in a Subpopulation of Mouse Retinal Ganglion Cells.Front Cell Neurosci. 2018 Oct 16;12:353. doi: 10.3389/fncel.2018.00353. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30386208 Free PMC article.
-
Structural basis of TRPV5 channel inhibition by econazole revealed by cryo-EM.Nat Struct Mol Biol. 2018 Jan;25(1):53-60. doi: 10.1038/s41594-017-0009-1. Epub 2018 Jan 1. Nat Struct Mol Biol. 2018. PMID: 29323279 Free PMC article.
-
Effect of SKF‑96365 on cardiomyocyte hypertrophy induced by angiotensin II.Mol Med Rep. 2020 Feb;21(2):806-814. doi: 10.3892/mmr.2019.10877. Epub 2019 Dec 11. Mol Med Rep. 2020. PMID: 31974621 Free PMC article.
References
-
- Al-Ansary D, Bogeski I, Disteldorf BM, Becherer U, Niemeyer BA. ATP modulates Ca2+ uptake by TRPV6 and is counteracted by isoform-specific phosphorylation. FASEB J. 2010;24:425–435. - PubMed
-
- Baez-Nieto D, Castillo JP, Dragicevic C, Alvarez O, Latorre R. Thermo-TRP channels: biophysics of polymodal receptors. Adv Exp Med Biol. 2011;704:469–490. - PubMed
-
- Bang S, Kim KY, Yoo S, Lee SH, Hwang SW. Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci Lett. 2007;425:120–125. - PubMed
-
- Benlekbir S, Bueler SA, Rubinstein JL. Structure of the vacuolar-type ATPase from Saccharomyces cerevisiae at 11- Å resolution. Nat Struct Mol Biol. 2012;19:1356–1362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

