Inhibition of the inflammatory cytokine tumor necrosis factor-alpha with etanercept provides protection against lethal H1N1 influenza infection in mice
- PMID: 24373231
- PMCID: PMC4057515
- DOI: 10.1186/cc13171
Inhibition of the inflammatory cytokine tumor necrosis factor-alpha with etanercept provides protection against lethal H1N1 influenza infection in mice
Abstract
Introduction: Factors implicated in influenza-mediated morbidity and mortality include robust cytokine production (cytokine storm), excessive inflammatory infiltrates, and virus-induced tissue destruction. Tumor necrosis factor-alpha (TNF-α) is an important pro-inflammatory cytokine present during influenza infection, but it is unclear whether direct inhibition of TNF-α can elicit protection against influenza infection.
Methods: In this study, the commercially available TNF-α inhibitor etanercept was used to inhibit TNF-α induced by lethal A/FM/1/47 (H1N1) influenza virus infection of mice. The effects of TNF-α inhibition on mouse survival, pathologic changes, immune cell infiltration, inflammatory cytokine secretion, Toll-like receptor expression, and activation of the NF-κB (nuclear factor kappa B) signaling pathway were evaluated.
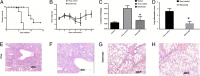
Results: The intranasal delivery of etanercept provided significant protection against mortality (30% of mice survived up to 14 days after infection) in mice treated with etanercept. In contrast, no survivors were found beyond 6 days in mice treated with saline after lethal challenge with H1N1 influenza virus. It was observed that etanercept significantly reduced inflammatory cell infiltration (for example, macrophages and neutrophils), inflammatory cytokine secretion (for example, interleukin-6, TNF-α, and interferon gamma), and expression of Toll-like receptors (TLR-3, TLR-4, and TLR-7). Etanercept also downregulated and inhibited the cascade proteins of the NF-κB signaling pathway (for example, MyD88, TRIF, NF-κB, and p65), as well as enhanced host control of virus replication.
Conclusions: These findings indicate that etanercept, by blocking TNF-α, can significantly downregulate excessive inflammatory immune responses and provide protection against lethal influenza infection, making its use a novel strategy for controlling severe influenza-induced viral pneumonia.
Figures
Similar articles
-
Flavonoids from Houttuynia cordata attenuate H1N1-induced acute lung injury in mice via inhibition of influenza virus and Toll-like receptor signalling.Phytomedicine. 2020 Feb;67:153150. doi: 10.1016/j.phymed.2019.153150. Epub 2019 Dec 16. Phytomedicine. 2020. PMID: 31958713
-
Liu Shen Wan inhibits influenza a virus and excessive virus-induced inflammatory response via suppression of TLR4/NF-κB signaling pathway in vitro and in vivo.J Ethnopharmacol. 2020 Apr 24;252:112584. doi: 10.1016/j.jep.2020.112584. Epub 2020 Jan 21. J Ethnopharmacol. 2020. PMID: 31972325
-
Targeting ectromelia virus and TNF/NF-κB or STAT3 signaling for effective treatment of viral pneumonia.Proc Natl Acad Sci U S A. 2022 Feb 22;119(8):e2112725119. doi: 10.1073/pnas.2112725119. Proc Natl Acad Sci U S A. 2022. PMID: 35177474 Free PMC article.
-
Quelling the storm: utilization of sphingosine-1-phosphate receptor signaling to ameliorate influenza virus-induced cytokine storm.Immunol Res. 2011 Oct;51(1):15-25. doi: 10.1007/s12026-011-8240-z. Immunol Res. 2011. PMID: 21901448 Review.
-
The role of tumor necrosis factor alpha in the pathophysiology of congestive heart failure.Curr Cardiol Rep. 2000 May;2(3):189-97. doi: 10.1007/s11886-000-0068-4. Curr Cardiol Rep. 2000. PMID: 10980892 Review.
Cited by
-
Etanercept Protected Against Cigarette Smoke Extract-Induced Inflammation and Apoptosis of Human Pulmonary Artery Endothelial Cells via Regulating TNFR1.Int J Chron Obstruct Pulmon Dis. 2021 May 11;16:1329-1345. doi: 10.2147/COPD.S295580. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 34007171 Free PMC article.
-
Perispinal Delivery of CNS Drugs.CNS Drugs. 2016 Jun;30(6):469-80. doi: 10.1007/s40263-016-0339-2. CNS Drugs. 2016. PMID: 27120182 Free PMC article. Review.
-
Therapeutic Targeting of Inflammation and Virus Simultaneously Ameliorates Influenza Pneumonia and Protects from Morbidity and Mortality.Viruses. 2023 Jan 23;15(2):318. doi: 10.3390/v15020318. Viruses. 2023. PMID: 36851532 Free PMC article.
-
Mitochondrial disease disrupts hepatic allostasis and lowers the threshold for immune-mediated liver toxicity.Mol Metab. 2020 Jul;37:100981. doi: 10.1016/j.molmet.2020.100981. Epub 2020 Mar 26. Mol Metab. 2020. PMID: 32283081 Free PMC article.
-
Beneficial and Detrimental Effects of Cytokines during Influenza and COVID-19.Viruses. 2024 Feb 18;16(2):308. doi: 10.3390/v16020308. Viruses. 2024. PMID: 38400083 Free PMC article. Review.
References
-
- Liu D, Shi W, Shi Y, Wang D, Xiao H, Li W, Bi Y, Wu Y, Li X, Yan J, Liu W, Zhao G, Yang W, Wang Y, Ma J, Shu Y, Lei F, Gao GF. Origin and diversity of novel avian influenza A H7N9 viruses causing human infection: phylogenetic, structural, and coalescent analyses. Lancet. 2013;17:1926–1932. doi: 10.1016/S0140-6736(13)60938-1. - DOI - PubMed
-
- Dai C, Jiang M. Understanding H7N9 avian flu. BMJ. 2013. doi:10.1136/bmj.f2755. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

