Therapeutic MSC exosomes are derived from lipid raft microdomains in the plasma membrane
- PMID: 24371518
- PMCID: PMC3873122
- DOI: 10.3402/jev.v2i0.22614
Therapeutic MSC exosomes are derived from lipid raft microdomains in the plasma membrane
Abstract
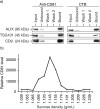
Background: Mesenchymal stem cell (MSC) was previously shown to secrete lipid vesicles that when purified by high performance liquid chromatography as a population of homogenously sized particles with a hydrodynamic radius of 55-65 nm reduce infarct size in a mouse model of myocardial ischemia/reperfusion injury. As these vesicles exhibit many biophysical and biochemical properties of exosomes, they were identified as exosomes. Here we investigated if these lipid vesicles were indeed exosomes that have an endosomal biogenesis.
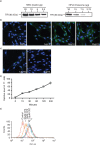
Method: In most cells, endocytosis is thought to occur at specialized microdomains known as lipid rafts. To demonstrate an endosomal origin for MSC exosomes, MSCs were pulsed with ligands e.g. transferrin (Tfs) and Cholera Toxin B (CTB) that bind receptors in lipid rafts. The endocytosed ligands were then chased to determine if they were incorporated into the exosomes.
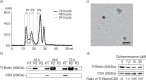
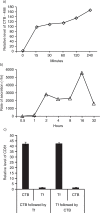
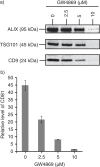
Results: A fraction of exogenous Tfs was found to recycle into MSC exosomes. When MSCs were pulsed with labelled Tfs in the presence of chlorpromazine, an inhibitor of clathrin-mediated endocytosis, Tf incorporation in CD81-immunoprecipitate was reduced during the chase. CTB which binds GM1 gangliosides that are enriched in lipid rafts extracted exosome-associated proteins, CD81, CD9, Alix and Tsg101 from MSC-conditioned medium. Exogenous CTBs were pulse-chased into secreted vesicles. Extraction of Tf- or CTB-binding vesicles in an exosome preparation mutually depleted each other. Inhibition of sphingomyelinases reduced CTB-binding vesicles.
Conclusion: Together, our data demonstrated that MSC exosomes are derived from endocytosed lipid rafts and that their protein cargo includes exosome-associated proteins CD81, CD9, Alix and Tsg101.
Keywords: cholera toxin B; clathrin mediated endocytosis; exosomes; lipid rafts; mesenchymal stem cells; transferrin.
Figures
Similar articles
-
Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury.Stem Cell Res. 2010 May;4(3):214-22. doi: 10.1016/j.scr.2009.12.003. Epub 2010 Jan 4. Stem Cell Res. 2010. PMID: 20138817
-
MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA.J Extracell Vesicles. 2016 Feb 24;5:29828. doi: 10.3402/jev.v5.29828. eCollection 2016. J Extracell Vesicles. 2016. PMID: 26928672 Free PMC article.
-
MSC exosome works through a protein-based mechanism of action.Biochem Soc Trans. 2018 Aug 20;46(4):843-853. doi: 10.1042/BST20180079. Epub 2018 Jul 9. Biochem Soc Trans. 2018. PMID: 29986939 Free PMC article. Review.
-
Membrane lipids define small extracellular vesicle subtypes secreted by mesenchymal stromal cells.J Lipid Res. 2019 Feb;60(2):318-322. doi: 10.1194/jlr.R087411. Epub 2018 Aug 28. J Lipid Res. 2019. PMID: 30154233 Free PMC article. Review.
-
Cholera toxin entry into pig enterocytes occurs via a lipid raft- and clathrin-dependent mechanism.Biochemistry. 2005 Jan 25;44(3):873-82. doi: 10.1021/bi047959+. Biochemistry. 2005. PMID: 15654743
Cited by
-
Cargo and cell-specific differences in extracellular vesicle populations identified by multiplexed immunofluorescent analysis.J Extracell Vesicles. 2020 Jul 17;9(1):1789326. doi: 10.1080/20013078.2020.1789326. J Extracell Vesicles. 2020. PMID: 32944176 Free PMC article.
-
Avoiding false positive antigen detection by flow cytometry on blood cell derived microparticles: the importance of an appropriate negative control.PLoS One. 2015 May 15;10(5):e0127209. doi: 10.1371/journal.pone.0127209. eCollection 2015. PLoS One. 2015. PMID: 25978814 Free PMC article.
-
Extracellular vesicles yield predictive pre-eclampsia biomarkers.J Extracell Vesicles. 2017 Dec 13;6(1):1408390. doi: 10.1080/20013078.2017.1408390. eCollection 2017. J Extracell Vesicles. 2017. PMID: 29296254 Free PMC article.
-
Role of exosome-associated microRNA in diagnostic and therapeutic applications to metabolic disorders.J Zhejiang Univ Sci B. 2018 Mar.;19(3):183-198. doi: 10.1631/jzus.B1600490. J Zhejiang Univ Sci B. 2018. PMID: 29504312 Free PMC article. Review.
-
Salivary Exosomes: Emerging Roles in Systemic Disease.Int J Biol Sci. 2018 Apr 30;14(6):633-643. doi: 10.7150/ijbs.25018. eCollection 2018. Int J Biol Sci. 2018. PMID: 29904278 Free PMC article. Review.
References
-
- Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–93. - PubMed
-
- Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. - PubMed
-
- Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–42. - PubMed
-
- In't Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548–9. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

