Deletion of Sirt3 does not affect atherosclerosis but accelerates weight gain and impairs rapid metabolic adaptation in LDL receptor knockout mice: implications for cardiovascular risk factor development
- PMID: 24370889
- PMCID: PMC3898152
- DOI: 10.1007/s00395-013-0399-0
Deletion of Sirt3 does not affect atherosclerosis but accelerates weight gain and impairs rapid metabolic adaptation in LDL receptor knockout mice: implications for cardiovascular risk factor development
Abstract
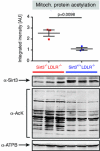
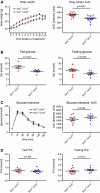
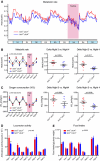
Sirt3 is a mitochondrial NAD(+)-dependent deacetylase that governs mitochondrial metabolism and reactive oxygen species homeostasis. Sirt3 deficiency has been reported to accelerate the development of the metabolic syndrome. However, the role of Sirt3 in atherosclerosis remains enigmatic. We aimed to investigate whether Sirt3 deficiency affects atherosclerosis, plaque vulnerability, and metabolic homeostasis. Low-density lipoprotein receptor knockout (LDLR(-/-)) and LDLR/Sirt3 double-knockout (Sirt3(-/-)LDLR(-/-)) mice were fed a high-cholesterol diet (1.25 % w/w) for 12 weeks. Atherosclerosis was assessed en face in thoraco-abdominal aortae and in cross sections of aortic roots. Sirt3 deletion led to hepatic mitochondrial protein hyperacetylation. Unexpectedly, though plasma malondialdehyde levels were elevated in Sirt3-deficient mice, Sirt3 deletion affected neither plaque burden nor features of plaque vulnerability (i.e., fibrous cap thickness and necrotic core diameter). Likewise, plaque macrophage and T cell infiltration as well as endothelial activation remained unaltered. Electron microscopy of aortic walls revealed no difference in mitochondrial microarchitecture between both groups. Interestingly, loss of Sirt3 was associated with accelerated weight gain and an impaired capacity to cope with rapid changes in nutrient supply as assessed by indirect calorimetry. Serum lipid levels and glucose tolerance were unaffected by Sirt3 deletion in LDLR(-/-) mice. Sirt3 deficiency does not affect atherosclerosis in LDLR(-/-) mice. However, Sirt3 controls systemic levels of oxidative stress, limits expedited weight gain, and allows rapid metabolic adaptation. Thus, Sirt3 may contribute to postponing cardiovascular risk factor development.
Figures

Similar articles
-
Mild endothelial dysfunction in Sirt3 knockout mice fed a high-cholesterol diet: protective role of a novel C/EBP-β-dependent feedback regulation of SOD2.Basic Res Cardiol. 2016 May;111(3):33. doi: 10.1007/s00395-016-0552-7. Epub 2016 Apr 12. Basic Res Cardiol. 2016. PMID: 27071400 Free PMC article.
-
Nuclear factor E2-related factor 2 deficiency impairs atherosclerotic lesion development but promotes features of plaque instability in hypercholesterolaemic mice.Cardiovasc Res. 2019 Jan 1;115(1):243-254. doi: 10.1093/cvr/cvy143. Cardiovasc Res. 2019. PMID: 29917052
-
The effects of diet on occlusive coronary artery atherosclerosis and myocardial infarction in scavenger receptor class B, type 1/low-density lipoprotein receptor double knockout mice.Arterioscler Thromb Vasc Biol. 2014 Nov;34(11):2394-403. doi: 10.1161/ATVBAHA.114.304200. Epub 2014 Sep 11. Arterioscler Thromb Vasc Biol. 2014. PMID: 25212235
-
Muscle or liver-specific Sirt3 deficiency induces hyperacetylation of mitochondrial proteins without affecting global metabolic homeostasis.Sci Rep. 2012;2:425. doi: 10.1038/srep00425. Epub 2012 May 28. Sci Rep. 2012. PMID: 22645641 Free PMC article.
-
Sirtuin-3-Mediated Cellular Metabolism Links Cardiovascular Remodeling with Hypertension.Biology (Basel). 2023 May 6;12(5):686. doi: 10.3390/biology12050686. Biology (Basel). 2023. PMID: 37237500 Free PMC article. Review.
Cited by
-
Targeting histone deacetylases: perspectives for epigenetic-based therapy in cardio-cerebrovascular disease.J Geriatr Cardiol. 2015 Mar;12(2):153-64. doi: 10.11909/j.issn.1671-5411.2015.02.010. J Geriatr Cardiol. 2015. PMID: 25870619 Free PMC article. Review.
-
Targeting epigenetics and non-coding RNAs in atherosclerosis: from mechanisms to therapeutics.Pharmacol Ther. 2019 Apr;196:15-43. doi: 10.1016/j.pharmthera.2018.11.003. Epub 2018 Nov 13. Pharmacol Ther. 2019. PMID: 30439455 Free PMC article.
-
SIRT3 deficiency exacerbates ischemia-reperfusion injury: implication for aged hearts.Am J Physiol Heart Circ Physiol. 2014 Jun 15;306(12):H1602-9. doi: 10.1152/ajpheart.00027.2014. Epub 2014 Apr 18. Am J Physiol Heart Circ Physiol. 2014. PMID: 24748594 Free PMC article.
-
Sirtuin 3 deficiency does not alter host defenses against bacterial and fungal infections.Sci Rep. 2017 Jun 20;7(1):3853. doi: 10.1038/s41598-017-04263-x. Sci Rep. 2017. PMID: 28634345 Free PMC article.
-
Mild endothelial dysfunction in Sirt3 knockout mice fed a high-cholesterol diet: protective role of a novel C/EBP-β-dependent feedback regulation of SOD2.Basic Res Cardiol. 2016 May;111(3):33. doi: 10.1007/s00395-016-0552-7. Epub 2016 Apr 12. Basic Res Cardiol. 2016. PMID: 27071400 Free PMC article.
References
-
- Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WPT, Loria CM, Smith SC. Harmonizing the Metabolic Syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. - DOI - PubMed
-
- Bellizzi D, Rose G, Cavalcante P, Covello G, Dato S, De Rango F, Greco V, Magiolini M, Feraco E, Mari V, Franceschi C, Passarino G, De Benidictis G. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics. 2005;85:258–263. doi: 10.1016/j.ygeno.2004.11.003. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

