Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double-blind, double-dummy study
- PMID: 24361112
- PMCID: PMC4643396
- DOI: 10.1016/S1474-4422(13)70293-X
Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double-blind, double-dummy study
Erratum in
- Lancet Neurol. 2014 Mar;13(3):240
Abstract
Background: Levodopa is the most effective therapy for Parkinson's disease, but chronic treatment is associated with the development of potentially disabling motor complications. Experimental studies suggest that motor complications are due to non-physiological, intermittent administration of the drug, and can be reduced with continuous delivery. We aimed to assess efficacy and safety of levodopa-carbidopa intestinal gel delivered continuously through an intrajejunal percutaneous tube.
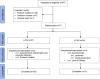
Methods: In our 12-week, randomised, double-blind, double-dummy, double-titration trial, we enrolled adults (aged ≥ 30 years) with advanced Parkinson's disease and motor complications at 26 centres in Germany, New Zealand, and the USA. Eligible participants had jejunal placement of a percutaneous gastrojejunostomy tube, and were then randomly allocated (1:1) to treatment with immediate-release oral levodopa-carbidopa plus placebo intestinal gel infusion or levodopa-carbidopa intestinal gel infusion plus oral placebo. Randomisation was stratified by site, with a mixed block size of 2 or 4. The primary endpoint was change from baseline to final visit in motor off-time. We assessed change in motor on-time without troublesome dyskinesia as a prespecified key secondary outcome. We assessed efficacy in a full-analysis set of participants with data for baseline and at least one post-baseline assessment, and imputed missing data with the last observation carried forward approach. We assessed safety in randomly allocated patients who underwent the percutaneous gastrojejunostomy procedure. This study is registered with ClinicalTrials.gov, numbers NCT00660387 and NCT0357994.
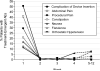
Findings: From baseline to 12 weeks in the full-analysis set, mean off-time decreased by 4.04 h (SE 0.65) for 35 patients allocated to the levodopa-carbidopa intestinal gel group compared with a decrease of 2.14 h (0.66) for 31 patients allocated to immediate-release oral levodopa-carbidopa (difference -1.91 h [95% CI -3.05 to -0.76]; p=0.0015). Mean on-time without troublesome dyskinesia increased by 4.11 h (SE 0.75) in the intestinal gel group and 2.24 h (0.76) in the immediate-release oral group (difference 1.86 [95% CI 0.56 to 3.17]; p=0.0059). In the safety analyses 35 (95%) of 37 patients allocated to the levodopa-carbidopa intestinal gel group had adverse events (five [14%] serious), as did 34 (100%) of 34 patients allocated to the immediate-release oral levodopa-carbidopa group (seven [21%] serious), mainly associated with the percutaneous gastrojejunostomy tube.
Interpretation: Continuous delivery of levodopa-carbidopa with an intestinal gel offers a promising option for control of advanced Parkinson's disease with motor complications. Benefits noted with intestinal gel delivery were of a greater magnitude than were those obtained with medical therapies to date, and our study is, to our knowledge, the first demonstration of the benefit of continuous levodopa delivery in a double-blind controlled study.
Funding: AbbVie.
Trial registration: ClinicalTrials.gov NCT00660387 NCT00357994 NCT00660387.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Jejunal levodopa infusion in Parkinson's disease.Lancet Neurol. 2014 Feb;13(2):128-9. doi: 10.1016/S1474-4422(13)70291-6. Epub 2013 Dec 20. Lancet Neurol. 2014. PMID: 24361113 No abstract available.
-
Parkinson disease: Intestinal levodopa infusion in PD--the first randomized trial.Nat Rev Neurol. 2014 Mar;10(3):128-9. doi: 10.1038/nrneurol.2014.26. Epub 2014 Feb 18. Nat Rev Neurol. 2014. PMID: 24535462 No abstract available.
-
Continuous infusion of levodopa-carbidopa intestinal gel in Parkinson's disease.J Comp Eff Res. 2014 Jul;3(4):331-3. doi: 10.2217/cer.14.33. J Comp Eff Res. 2014. PMID: 25275230
Similar articles
-
Safety and efficacy of continuous subcutaneous levodopa-carbidopa infusion (ND0612) for Parkinson's disease with motor fluctuations (BouNDless): a phase 3, randomised, double-blind, double-dummy, multicentre trial.Lancet Neurol. 2024 May;23(5):465-476. doi: 10.1016/S1474-4422(24)00052-8. Epub 2024 Mar 15. Lancet Neurol. 2024. PMID: 38499015 Clinical Trial.
-
Safety and efficacy of continuous subcutaneous foslevodopa-foscarbidopa in patients with advanced Parkinson's disease: a randomised, double-blind, active-controlled, phase 3 trial.Lancet Neurol. 2022 Dec;21(12):1099-1109. doi: 10.1016/S1474-4422(22)00400-8. Lancet Neurol. 2022. PMID: 36402160 Clinical Trial.
-
Effect of levodopa-carbidopa intestinal gel on dyskinesia in advanced Parkinson's disease patients.Mov Disord. 2016 Apr;31(4):530-7. doi: 10.1002/mds.26528. Epub 2016 Jan 28. Mov Disord. 2016. PMID: 26817533 Free PMC article. Clinical Trial.
-
Current Practices for Outpatient Initiation of Levodopa-Carbidopa Intestinal Gel for Management of Advanced Parkinson's Disease in the United States.Adv Ther. 2019 Sep;36(9):2233-2246. doi: 10.1007/s12325-019-01014-4. Epub 2019 Jul 5. Adv Ther. 2019. PMID: 31278691 Free PMC article. Review.
-
[Application of levodopa/carbidopa intestinal gel in advanced Parkinson's disease].Neuropsychopharmacol Hung. 2015 Dec;17(4):191-6. Neuropsychopharmacol Hung. 2015. PMID: 26727723 Review. Hungarian.
Cited by
-
Dyskinesia and Pain in Advanced Parkinson's Disease: Post Hoc Analysis from the Phase 3b, Open-Label, Randomized DYSCOVER Study.Neurol Ther. 2024 Apr;13(2):437-447. doi: 10.1007/s40120-024-00583-z. Epub 2024 Feb 12. Neurol Ther. 2024. PMID: 38345741 Free PMC article.
-
Decreased hepatic enzymes reflect the decreased vitamin B6 levels in Parkinson's disease patients.Pharmacol Res Perspect. 2024 Feb;12(1):e1174. doi: 10.1002/prp2.1174. Pharmacol Res Perspect. 2024. PMID: 38287715 Free PMC article.
-
Monotherapy with infusion therapies - useful or not?J Neural Transm (Vienna). 2024 Nov;131(11):1341-1348. doi: 10.1007/s00702-024-02801-2. Epub 2024 Jul 5. J Neural Transm (Vienna). 2024. PMID: 38967810 Review.
-
From medications to surgery: advances in the treatment of motor complications in Parkinson's disease.Drugs Context. 2019 Aug 13;8:212592. doi: 10.7573/dic.212592. eCollection 2019. Drugs Context. 2019. PMID: 31516532 Free PMC article. Review.
-
Prevalence of Advanced Parkinson's Disease in Patients Treated in the Hospitals of the Spanish National Healthcare System: The PARADISE Study.Brain Sci. 2021 Nov 24;11(12):1557. doi: 10.3390/brainsci11121557. Brain Sci. 2021. PMID: 34942858 Free PMC article.
References
-
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–458. - PubMed
-
- Olanow CW, Stern MB, Sethi K. Scientific and Clinical Basis for the treatment of PD – 2009. Neurology. 2009;72(21 Suppl 4):S1–136. - PubMed
-
- The Deep Brain Stimulation for PD study group Deep brain stimulation of the subthalamic nucleus or globus pallidus pars interna in Parkinson's disease. New Eng J Med. 2001;345:956–963. - PubMed
-
- Fox SH, Katzenschlager R, Lim SY, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the motor symptoms of Parkinson's disease. Mov Disord. 2011;26(Suppl 3):S2–41. - PubMed
-
- Olanow CW, Obeso JA, Stocchi F. Continuous Dopamine Receptor Stimulation in the Treatment of Parkinson's Disease: Scientific Rationale and Clinical Implications. Lancet Neurology. 2006;5:677–687. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- K08 NS060948/NS/NINDS NIH HHS/United States
- R01 NS064934/NS/NINDS NIH HHS/United States
- F31 NS076017/NS/NINDS NIH HHS/United States
- U18 NS082132/NS/NINDS NIH HHS/United States
- U10 NS044547/NS/NINDS NIH HHS/United States
- F30 NS065661/NS/NINDS NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- K01 NS069614/NS/NINDS NIH HHS/United States
- R25 NS079188/NS/NINDS NIH HHS/United States
- F31 NS081963/NS/NINDS NIH HHS/United States
- R01 MH082304/MH/NIMH NIH HHS/United States
- K23 MH092735/MH/NIMH NIH HHS/United States
- R01 NS037406/NS/NINDS NIH HHS/United States
- K23 NS080912/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

