MicroRNA-339-5p down-regulates protein expression of β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) in human primary brain cultures and is reduced in brain tissue specimens of Alzheimer disease subjects
- PMID: 24352696
- PMCID: PMC3931075
- DOI: 10.1074/jbc.M113.518241
MicroRNA-339-5p down-regulates protein expression of β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) in human primary brain cultures and is reduced in brain tissue specimens of Alzheimer disease subjects
Abstract
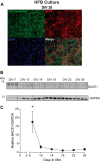
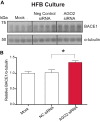
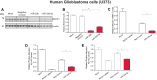
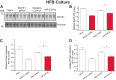
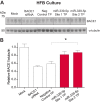
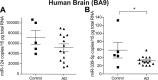
Alzheimer disease (AD) results, in part, from the excess accumulation of the amyloid-β (Aβ) peptide as neuritic plaques in the brain. The short Aβ peptide is derived from the large transmembrane Aβ precursor protein (APP). The rate-limiting step in the production of Aβ from APP is mediated by the β-site APP-cleaving enzyme 1 (BACE1). Dysregulation of BACE1 levels leading to excess Aβ deposition is implicated in sporadic AD. Thus, elucidating the full complement of regulatory pathways that control BACE1 expression is key to identifying novel drug targets central to the Aβ-generating process. MicroRNAs (miRNAs) are expected to participate in this molecular network. Here, we identified a known miRNA, miR-339-5p, as a key contributor to this regulatory network. Two distinct miR-339-5p target sites were predicted in the BACE1 3'-UTR by in silico analyses. Co-transfection of miR-339-5p with a BACE1 3'-UTR reporter construct resulted in significant reduction in reporter expression. Mutation of both target sites eliminated this effect. Delivery of the miR-339-5p mimic also significantly inhibited expression of BACE1 protein in human glioblastoma cells and human primary brain cultures. Delivery of target protectors designed against the miR-339-5p BACE1 3'-UTR target sites in primary human brain cultures significantly elevated BACE1 expression. Finally, miR-339-5p levels were found to be significantly reduced in brain specimens isolated from AD patients as compared with age-matched controls. Therefore, miR-339-5p regulates BACE1 expression in human brain cells and is most likely dysregulated in at least a subset of AD patients making this miRNA a novel drug target.
Keywords: Aging; Alzheimer Disease; Dementia; Gene Regulation; Human Brain Tissue; Human Neuron; MicroRNA; Noncoding RNA; Secretases; β-Peptide.
Figures


Similar articles
-
Circulating Small Extracellular Vesicle-Derived miR-342-5p Ameliorates Beta-Amyloid Formation via Targeting Beta-site APP Cleaving Enzyme 1 in Alzheimer's Disease.Cells. 2022 Nov 29;11(23):3830. doi: 10.3390/cells11233830. Cells. 2022. PMID: 36497090 Free PMC article.
-
miR-15b reduces amyloid-β accumulation in SH-SY5Y cell line through targetting NF-κB signaling and BACE1.Biosci Rep. 2018 Nov 13;38(6):BSR20180051. doi: 10.1042/BSR20180051. Print 2018 Dec 21. Biosci Rep. 2018. PMID: 29961672 Free PMC article.
-
miR-186 is decreased in aged brain and suppresses BACE1 expression.J Neurochem. 2016 May;137(3):436-45. doi: 10.1111/jnc.13507. Epub 2016 Mar 30. J Neurochem. 2016. PMID: 26710318 Free PMC article.
-
The beta-secretase, BACE: a prime drug target for Alzheimer's disease.J Mol Neurosci. 2001 Oct;17(2):157-70. doi: 10.1385/JMN:17:2:157. J Mol Neurosci. 2001. PMID: 11816789 Review.
-
BACE1: the beta-secretase enzyme in Alzheimer's disease.J Mol Neurosci. 2004;23(1-2):105-14. doi: 10.1385/JMN:23:1-2:105. J Mol Neurosci. 2004. PMID: 15126696 Review.
Cited by
-
In silico Analysis of Polymorphisms in microRNAs Deregulated in Alzheimer Disease.Front Neurosci. 2021 Mar 24;15:631852. doi: 10.3389/fnins.2021.631852. eCollection 2021. Front Neurosci. 2021. PMID: 33841080 Free PMC article.
-
miR-339-5p Increases Radiosensitivity of Lung Cancer Cells by Targeting Phosphatases of Regenerating Liver-1 (PRL-1).Med Sci Monit. 2018 Nov 21;24:8408-8416. doi: 10.12659/MSM.910808. Med Sci Monit. 2018. PMID: 30462625 Free PMC article.
-
Microglia secrete miR-146a-5p-containing exosomes to regulate neurogenesis in depression.Mol Ther. 2022 Mar 2;30(3):1300-1314. doi: 10.1016/j.ymthe.2021.11.006. Epub 2021 Nov 10. Mol Ther. 2022. PMID: 34768001 Free PMC article.
-
Getting miRNA Therapeutics into the Target Cells for Neurodegenerative Diseases: A Mini-Review.Front Mol Neurosci. 2016 Nov 22;9:129. doi: 10.3389/fnmol.2016.00129. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27920668 Free PMC article. Review.
-
Epigenetics of Alzheimer's Disease.Biomolecules. 2021 Jan 30;11(2):195. doi: 10.3390/biom11020195. Biomolecules. 2021. PMID: 33573255 Free PMC article. Review.
References
-
- Thies W., Bleiler L., and Alzheimer's Association (2013) 2013 Alzheimer's disease facts and figures. Alzheimers Dement. 9, 208–245 - PubMed
-
- Lahiri D. K. (2012) Prions: a piece of the puzzle? Science 337, 1172. - PubMed
-
- Hardy J., Selkoe D. J. (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 - PubMed
-
- Karran E., Mercken M., De Strooper B. (2011) The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 10, 698–712 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

