Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity
- PMID: 24352444
- PMCID: PMC3958102
- DOI: 10.1128/JVI.03230-13
Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity
Abstract
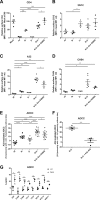
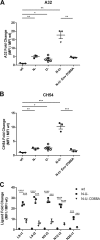
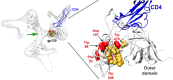
Anti-HIV-1 envelope glycoprotein (Env) antibodies without broadly neutralizing activity correlated with protection in the RV144 clinical trial, stimulating interest in other protective mechanisms involving antibodies, such as antibody-dependent cell-mediated cytotoxicity (ADCC). Env epitopes targeted by many antibodies effective at mediating ADCC are poorly exposed on the unliganded Env trimer. Here we investigated the mechanism of exposure of ADCC epitopes on Env and showed that binding of Env and CD4 within the same HIV-1-infected cell effectively exposes these epitopes. Env capacity to transit to the CD4-bound conformation is required for ADCC epitope exposure. Importantly, cell surface CD4 downregulation by Nef and Vpu accessory proteins and Vpu-mediated BST-2 antagonism modulate exposure of ADCC-mediating epitopes and reduce the susceptibility of infected cells to this effector function in vitro. Significantly, Env conformational changes induced by cell surface CD4 are conserved among Env from HIV-1 and HIV-2/SIVmac lineages. Altogether, our observations describe a highly conserved mechanism required to expose ADCC epitopes that might help explain the evolutionary advantage of downregulation of cell surface CD4 by the HIV-1 Vpu and Nef proteins.
Importance: HIV-1 envelope epitopes targeted by many antibodies effective at mediating antibody-dependent cell-mediated cytotoxicity (ADCC) are poorly exposed on the unliganded envelope trimer. Here we investigated the mechanism of exposure of these epitopes and found that envelope interaction with the HIV-1 CD4 receptor is required to expose some of these epitopes. Moreover, our results suggest that HIV-1 CD4 downregulation might help avoid the killing of HIV-1-infected cells by this immune mechanism.
Figures

Similar articles
-
BST-2 Expression Modulates Small CD4-Mimetic Sensitization of HIV-1-Infected Cells to Antibody-Dependent Cellular Cytotoxicity.J Virol. 2017 May 12;91(11):e00219-17. doi: 10.1128/JVI.00219-17. Print 2017 Jun 1. J Virol. 2017. PMID: 28331088 Free PMC article.
-
Incomplete Downregulation of CD4 Expression Affects HIV-1 Env Conformation and Antibody-Dependent Cellular Cytotoxicity Responses.J Virol. 2018 Jun 13;92(13):e00484-18. doi: 10.1128/JVI.00484-18. Print 2018 Jul 1. J Virol. 2018. PMID: 29669829 Free PMC article.
-
Co-receptor Binding Site Antibodies Enable CD4-Mimetics to Expose Conserved Anti-cluster A ADCC Epitopes on HIV-1 Envelope Glycoproteins.EBioMedicine. 2016 Oct;12:208-218. doi: 10.1016/j.ebiom.2016.09.004. Epub 2016 Sep 9. EBioMedicine. 2016. PMID: 27633463 Free PMC article.
-
Unlocking HIV-1 Env: implications for antibody attack.AIDS Res Ther. 2017 Sep 12;14(1):42. doi: 10.1186/s12981-017-0168-5. AIDS Res Ther. 2017. PMID: 28893275 Free PMC article. Review.
-
Impact of HIV-1 Envelope Conformation on ADCC Responses.Trends Microbiol. 2018 Apr;26(4):253-265. doi: 10.1016/j.tim.2017.10.007. Epub 2017 Nov 20. Trends Microbiol. 2018. PMID: 29162391 Review.
Cited by
-
CD4 mimetics sensitize HIV-1-infected cells to ADCC.Proc Natl Acad Sci U S A. 2015 May 19;112(20):E2687-94. doi: 10.1073/pnas.1506755112. Epub 2015 May 4. Proc Natl Acad Sci U S A. 2015. PMID: 25941367 Free PMC article.
-
Conserved molecular signatures in gp120 are associated with the genetic bottleneck during simian immunodeficiency virus (SIV), SIV-human immunodeficiency virus (SHIV), and HIV type 1 (HIV-1) transmission.J Virol. 2015 Apr;89(7):3619-29. doi: 10.1128/JVI.03235-14. Epub 2015 Jan 14. J Virol. 2015. PMID: 25589663 Free PMC article.
-
A New Family of Small-Molecule CD4-Mimetic Compounds Contacts Highly Conserved Aspartic Acid 368 of HIV-1 gp120 and Mediates Antibody-Dependent Cellular Cytotoxicity.J Virol. 2019 Nov 26;93(24):e01325-19. doi: 10.1128/JVI.01325-19. Print 2019 Dec 15. J Virol. 2019. PMID: 31554684 Free PMC article.
-
SOSIP Changes Affect Human Immunodeficiency Virus Type 1 Envelope Glycoprotein Conformation and CD4 Engagement.J Virol. 2018 Sep 12;92(19):e01080-18. doi: 10.1128/JVI.01080-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021898 Free PMC article.
-
Substitutions in Nef That Uncouple Tetherin and SERINC5 Antagonism Impair Simian Immunodeficiency Virus Replication in Primary Rhesus Macaque Lymphocytes.J Virol. 2022 Jun 8;96(11):e0017622. doi: 10.1128/jvi.00176-22. Epub 2022 May 10. J Virol. 2022. PMID: 35536019 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

