Nodular inflammatory foci are sites of T cell priming and control of murine cytomegalovirus infection in the neonatal lung
- PMID: 24348257
- PMCID: PMC3861546
- DOI: 10.1371/journal.ppat.1003828
Nodular inflammatory foci are sites of T cell priming and control of murine cytomegalovirus infection in the neonatal lung
Abstract
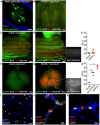
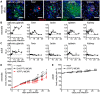
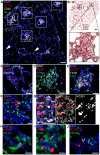
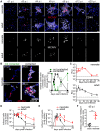
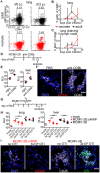
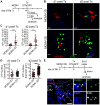
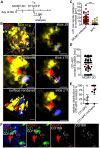
Neonates, including mice and humans, are highly susceptible to cytomegalovirus (CMV) infection. However, many aspects of neonatal CMV infections such as viral cell tropism, spatio-temporal distribution of the pathogen as well as genesis of antiviral immunity are unknown. With the use of reporter mutants of the murine cytomegalovirus (MCMV) we identified the lung as a primary target of mucosal infection in neonatal mice. Comparative analysis of neonatal and adult mice revealed a delayed control of virus replication in the neonatal lung mucosa explaining the pronounced systemic infection and disease in neonates. This phenomenon was supplemented by a delayed expansion of CD8(+) T cell clones recognizing the viral protein M45 in neonates. We detected viral infection at the single-cell level and observed myeloid cells forming "nodular inflammatory foci" (NIF) in the neonatal lung. Co-localization of infected cells within NIFs was associated with their disruption and clearance of the infection. By 2-photon microscopy, we characterized how neonatal antigen-presenting cells (APC) interacted with T cells and induced mature adaptive immune responses within such NIFs. We thus define NIFs of the neonatal lung as niches for prolonged MCMV replication and T cell priming but also as sites of infection control.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Control of primary mouse cytomegalovirus infection in lung nodular inflammatory foci by cooperation of interferon-gamma expressing CD4 and CD8 T cells.PLoS Pathog. 2018 Aug 28;14(8):e1007252. doi: 10.1371/journal.ppat.1007252. eCollection 2018 Aug. PLoS Pathog. 2018. PMID: 30153311 Free PMC article.
-
Cytomegalovirus inhibitors of programmed cell death restrict antigen cross-presentation in the priming of antiviral CD8 T cells.PLoS Pathog. 2024 Aug 15;20(8):e1012173. doi: 10.1371/journal.ppat.1012173. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39146364 Free PMC article.
-
Persistent viral replication and the development of T-cell responses after intranasal infection by MCMV.Med Microbiol Immunol. 2019 Aug;208(3-4):457-468. doi: 10.1007/s00430-019-00589-7. Epub 2019 Mar 8. Med Microbiol Immunol. 2019. PMID: 30848361 Free PMC article.
-
Cytomegalovirus (CMV) Pneumonitis: Cell Tropism, Inflammation, and Immunity.Int J Mol Sci. 2019 Aug 8;20(16):3865. doi: 10.3390/ijms20163865. Int J Mol Sci. 2019. PMID: 31398860 Free PMC article. Review.
-
Refining human T-cell immunotherapy of cytomegalovirus disease: a mouse model with 'humanized' antigen presentation as a new preclinical study tool.Med Microbiol Immunol. 2016 Dec;205(6):549-561. doi: 10.1007/s00430-016-0471-0. Epub 2016 Aug 18. Med Microbiol Immunol. 2016. PMID: 27539576 Review.
Cited by
-
Mck2-dependent infection of alveolar macrophages promotes replication of MCMV in nodular inflammatory foci of the neonatal lung.Mucosal Immunol. 2015 Jan;8(1):57-67. doi: 10.1038/mi.2014.42. Epub 2014 Jun 4. Mucosal Immunol. 2015. PMID: 24894498
-
Mast cells expedite control of pulmonary murine cytomegalovirus infection by enhancing the recruitment of protective CD8 T cells to the lungs.PLoS Pathog. 2014 Apr 24;10(4):e1004100. doi: 10.1371/journal.ppat.1004100. eCollection 2014 Apr. PLoS Pathog. 2014. PMID: 24763809 Free PMC article.
-
Alveolar Macrophages Are a Prominent but Nonessential Target for Murine Cytomegalovirus Infecting the Lungs.J Virol. 2015 Dec 30;90(6):2756-66. doi: 10.1128/JVI.02856-15. J Virol. 2015. PMID: 26719275 Free PMC article.
-
Type I Interferon Released by Myeloid Dendritic Cells Reversibly Impairs Cytomegalovirus Replication by Inhibiting Immediate Early Gene Expression.J Virol. 2015 Oct;89(19):9886-95. doi: 10.1128/JVI.01459-15. Epub 2015 Jul 22. J Virol. 2015. PMID: 26202227 Free PMC article.
-
Inducible Bronchus-Associated Lymphoid Tissue: Taming Inflammation in the Lung.Front Immunol. 2016 Jun 30;7:258. doi: 10.3389/fimmu.2016.00258. eCollection 2016. Front Immunol. 2016. PMID: 27446088 Free PMC article. Review.
References
-
- Cannon MJ, Schmid DS, Hyde TB (2010) Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 20: 202–213. - PubMed
-
- Weller TH (1971) The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. II. N Engl J Med 285: 267–274. - PubMed
-
- Britt W (2008) Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr Top Microbiol Immunol 325: 417–470. - PubMed
-
- Kenneson A, Cannon MJ (2007) Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 17: 253–276. - PubMed
-
- Mocarski ES, Shenk T, Pass R (2007) Cytomegalovirus. In: Knipe DM, Howley PM, editors. Fields Virology. 5th edition: Lippincott Williams & Wilkins. pp. 2702–2772.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

