Mesenchymal-transitioned cancer cells instigate the invasion of epithelial cancer cells through secretion of WNT3 and WNT5B
- PMID: 24344732
- PMCID: PMC4317934
- DOI: 10.1111/cas.12336
Mesenchymal-transitioned cancer cells instigate the invasion of epithelial cancer cells through secretion of WNT3 and WNT5B
Abstract
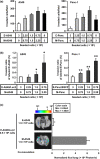
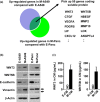
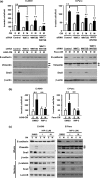
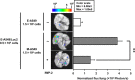
Although the heterogeneities of epithelial and mesenchymal-transitioned cancer cells are often observed within the tumor microenvironment, the biological significance of the interaction between epithelial cancer cells and mesenchymal-transitioned cancer cells is not yet understood. In this study, we show that the mesenchymal-transitioned cancer cells instigate the invasive ability and metastatic potential of the neighboring epithelial cancer cells in vitro and in vivo. We identify WNT3 and WNT5B as critical factors secreted from Transforming growth factor-induced mesenchymal cancer cells for instigating the epithelial cancer cell invasion along with the induction of secondary EMT phenotype. These results shed light on the significance of cancer heterogeneity and the interaction between epithelial and mesenchymal-transitioned cancer cells within the tumor microenvironment in promoting metastatic disease through the WNT-dependent mechanism.
Keywords: Cross-talk; epithelial-to-mesenchymal transition; heterogeneity; metastasis WNT.
© 2013 The Authors. Cancer Science published by Wiley Publishing Asia Pty Ltd on behalf of Japanese Cancer Association.
Figures

Similar articles
-
Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells.J Clin Invest. 2012 May;122(5):1849-68. doi: 10.1172/JCI59218. Epub 2012 Apr 16. J Clin Invest. 2012. PMID: 22505459 Free PMC article.
-
Epithelial-to-mesenchymal transition markers to predict response of Berberine in suppressing lung cancer invasion and metastasis.J Transl Med. 2014 Jan 24;12:22. doi: 10.1186/1479-5876-12-22. J Transl Med. 2014. PMID: 24456611 Free PMC article.
-
A Wnt-Induced Phenotypic Switch in Cancer-Associated Fibroblasts Inhibits EMT in Colorectal Cancer.Cancer Res. 2020 Dec 15;80(24):5569-5582. doi: 10.1158/0008-5472.CAN-20-0263. Epub 2020 Oct 14. Cancer Res. 2020. PMID: 33055221
-
Hepatocyte nuclear factor 6 suppresses the migration and invasive growth of lung cancer cells through p53 and the inhibition of epithelial-mesenchymal transition.J Biol Chem. 2013 Oct 25;288(43):31206-16. doi: 10.1074/jbc.M113.480285. Epub 2013 Sep 10. J Biol Chem. 2013. PMID: 24022481 Free PMC article.
-
Stereospecific effects of ginsenoside 20-Rg3 inhibits TGF-β1-induced epithelial-mesenchymal transition and suppresses lung cancer migration, invasion and anoikis resistance.Toxicology. 2014 Aug 1;322:23-33. doi: 10.1016/j.tox.2014.04.002. Epub 2014 May 2. Toxicology. 2014. PMID: 24793912
Cited by
-
P38 pathway as a key downstream signal of connective tissue growth factor to regulate metastatic potential in non-small-cell lung cancer.Cancer Sci. 2016 Oct;107(10):1416-1421. doi: 10.1111/cas.13009. Epub 2016 Sep 1. Cancer Sci. 2016. PMID: 27403934 Free PMC article.
-
TET1-mediated DNA hydroxymethylation activates inhibitors of the Wnt/β-catenin signaling pathway to suppress EMT in pancreatic tumor cells.J Exp Clin Cancer Res. 2019 Aug 9;38(1):348. doi: 10.1186/s13046-019-1334-5. J Exp Clin Cancer Res. 2019. PMID: 31399111 Free PMC article.
-
WNT5B in Physiology and Disease.Front Cell Dev Biol. 2021 May 4;9:667581. doi: 10.3389/fcell.2021.667581. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34017835 Free PMC article. Review.
-
Deconvolution of heterogeneous wound tissue samples into relative macrophage phenotype composition via models based on gene expression.Integr Biol (Camb). 2017 Apr 18;9(4):328-338. doi: 10.1039/c7ib00018a. Integr Biol (Camb). 2017. PMID: 28290581 Free PMC article.
-
COP9 signalosome subunit 5 regulates cancer metastasis by deubiquitinating SNAIL.Oncotarget. 2018 Apr 17;9(29):20670-20680. doi: 10.18632/oncotarget.25060. eCollection 2018 Apr 17. Oncotarget. 2018. PMID: 29755680 Free PMC article.
References
-
- Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. - PubMed
-
- Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12:323–34. - PubMed
-
- Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

