Fibroblasts in myocardial infarction: a role in inflammation and repair
- PMID: 24321195
- PMCID: PMC3995820
- DOI: 10.1016/j.yjmcc.2013.11.015
Fibroblasts in myocardial infarction: a role in inflammation and repair
Abstract
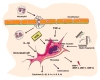
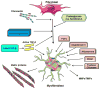
Fibroblasts do not only serve as matrix-producing reparative cells, but exhibit a wide range of functions in inflammatory and immune responses, angiogenesis and neoplasia. The adult mammalian myocardium contains abundant fibroblasts enmeshed within the interstitial and perivascular extracellular matrix. The current review manuscript discusses the dynamic phenotypic and functional alterations of cardiac fibroblasts following myocardial infarction. Extensive necrosis of cardiomyocytes in the infarcted heart triggers an intense inflammatory reaction. In the early stages of infarct healing, fibroblasts become pro-inflammatory cells, activating the inflammasome and producing cytokines, chemokines and proteases. Pro-inflammatory cytokines (such as Interleukin-1) delay myofibroblast transformation, until the wound is cleared from dead cells and matrix debris. Resolution of the inflammatory infiltrate is associated with fibroblast migration, proliferation, matrix protein synthesis and myofibroblast conversion. Growth factors and matricellular proteins play an important role in myofibroblast activation during the proliferative phase of healing. Formation of a mature cross-linked scar is associated with clearance of fibroblasts, as poorly-understood inhibitory signals restrain the fibrotic response. However, in the non-infarcted remodeling myocardium, local fibroblasts may remain activated in response to volume and pressure overload and may promote interstitial fibrosis. Considering their abundance, their crucial role in cardiac inflammation and repair, and their involvement in myocardial dysfunction and arrhythmogenesis, cardiac fibroblasts may be key therapeutic targets in cardiac remodeling. This article is part of a Special Issue entitled Myocyte-Fibroblast Signalling in Myocardium.
Keywords: Cardiac remodeling; Extracellular matrix; Fibroblast; Inflammation; Myocardial infarction; Transforming growth factor (TGF)-β.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Fibroblasts in post-infarction inflammation and cardiac repair.Biochim Biophys Acta. 2013 Apr;1833(4):945-53. doi: 10.1016/j.bbamcr.2012.08.023. Epub 2012 Sep 7. Biochim Biophys Acta. 2013. PMID: 22982064 Free PMC article. Review.
-
Cardiac fibroblast activation during myocardial infarction wound healing: Fibroblast polarization after MI.Matrix Biol. 2020 Sep;91-92:109-116. doi: 10.1016/j.matbio.2020.03.010. Epub 2020 May 21. Matrix Biol. 2020. PMID: 32446909 Free PMC article. Review.
-
The extracellular matrix modulates fibroblast phenotype and function in the infarcted myocardium.J Cardiovasc Transl Res. 2012 Dec;5(6):837-47. doi: 10.1007/s12265-012-9406-3. Epub 2012 Sep 7. J Cardiovasc Transl Res. 2012. PMID: 22956156 Free PMC article. Review.
-
Properties and Functions of Fibroblasts and Myofibroblasts in Myocardial Infarction.Cells. 2022 Apr 20;11(9):1386. doi: 10.3390/cells11091386. Cells. 2022. PMID: 35563692 Free PMC article. Review.
-
Fibroblasts and the extracellular matrix in right ventricular disease.Cardiovasc Res. 2017 Oct 1;113(12):1453-1464. doi: 10.1093/cvr/cvx146. Cardiovasc Res. 2017. PMID: 28957531 Free PMC article. Review.
Cited by
-
The Roles of Noncardiomyocytes in Cardiac Remodeling.Int J Biol Sci. 2020 Jul 2;16(13):2414-2429. doi: 10.7150/ijbs.47180. eCollection 2020. Int J Biol Sci. 2020. PMID: 32760209 Free PMC article. Review.
-
Post-ischemic cardioprotective potential of exogenous ubiquitin in myocardial remodeling late after ischemia/reperfusion injury.Life Sci. 2023 Jan 1;312:121216. doi: 10.1016/j.lfs.2022.121216. Epub 2022 Nov 24. Life Sci. 2023. PMID: 36435225 Free PMC article.
-
The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis.Circ Res. 2016 Jun 24;119(1):91-112. doi: 10.1161/CIRCRESAHA.116.303577. Circ Res. 2016. PMID: 27340270 Free PMC article. Review.
-
Adipose-Derived Mesenchymal Stem Cells-Derived Exosomes Carry MicroRNA-671 to Alleviate Myocardial Infarction Through Inactivating the TGFBR2/Smad2 Axis.Inflammation. 2021 Oct;44(5):1815-1830. doi: 10.1007/s10753-021-01460-9. Epub 2021 Apr 21. Inflammation. 2021. PMID: 33881681 Free PMC article.
-
The common characteristics and mutual effects of heart failure and atrial fibrillation: initiation, progression, and outcome of the two aging-related heart diseases.Heart Fail Rev. 2022 May;27(3):837-847. doi: 10.1007/s10741-021-10095-9. Epub 2021 Mar 25. Heart Fail Rev. 2022. PMID: 33768377 Review.
References
-
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–51. - PubMed
-
- Ito TK, Ishii G, Chiba H, Ochiai A. The VEGF angiogenic switch of fibroblasts is regulated by MMP-7 from cancer cells. Oncogene. 2007;26:7194–203. - PubMed
-
- Nag AC. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28:41–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

