TGF-β activation and function in immunity
- PMID: 24313777
- PMCID: PMC4010192
- DOI: 10.1146/annurev-immunol-032713-120257
TGF-β activation and function in immunity
Abstract
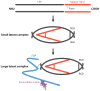
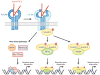
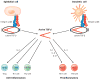
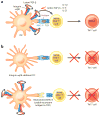
The cytokine TGF-β plays an integral role in regulating immune responses. TGF-β has pleiotropic effects on adaptive immunity, especially in the regulation of effector and regulatory CD4(+) T cell responses. Many immune and nonimmune cells can produce TGF-β, but it is always produced as an inactive complex that must be activated to exert functional effects. Thus, activation of latent TGF-β provides a crucial layer of regulation that controls TGF-β function. In this review, we highlight some of the important functional roles for TGF-β in immunity, focusing on its context-specific roles in either dampening or promoting T cell responses. We also describe how activation of TGF-β controls its function in the immune system, with a focus on the key roles for members of the integrin family in this process.
Figures

Similar articles
-
Revisiting the regulatory roles of the TGF-β family of cytokines.Autoimmun Rev. 2016 Sep;15(9):917-22. doi: 10.1016/j.autrev.2016.07.007. Epub 2016 Jul 5. Autoimmun Rev. 2016. PMID: 27392504 Review.
-
Intestinal dendritic cells specialize to activate transforming growth factor-β and induce Foxp3+ regulatory T cells via integrin αvβ8.Gastroenterology. 2011 Nov;141(5):1802-12. doi: 10.1053/j.gastro.2011.06.057. Epub 2011 Jun 30. Gastroenterology. 2011. PMID: 21723222 Free PMC article.
-
Regulation of TGFβ in the immune system: an emerging role for integrins and dendritic cells.Immunobiology. 2012 Dec;217(12):1259-65. doi: 10.1016/j.imbio.2012.06.009. Epub 2012 Jul 2. Immunobiology. 2012. PMID: 22902140 Free PMC article. Review.
-
Transforming Growth Factor-β Signaling in Immunity and Cancer.Immunity. 2019 Apr 16;50(4):924-940. doi: 10.1016/j.immuni.2019.03.024. Immunity. 2019. PMID: 30995507 Free PMC article. Review.
-
The TGF-β superfamily in dendritic cell biology.Cytokine Growth Factor Rev. 2015 Dec;26(6):647-57. doi: 10.1016/j.cytogfr.2015.06.002. Epub 2015 Jun 19. Cytokine Growth Factor Rev. 2015. PMID: 26115564 Review.
Cited by
-
Pulmonary resident memory T cells in respiratory virus infection and their inspiration on therapeutic strategies.Front Immunol. 2022 Aug 12;13:943331. doi: 10.3389/fimmu.2022.943331. eCollection 2022. Front Immunol. 2022. PMID: 36032142 Free PMC article. Review.
-
NAT10/ac4C/JunB facilitates TNBC malignant progression and immunosuppression by driving glycolysis addiction.J Exp Clin Cancer Res. 2024 Oct 4;43(1):278. doi: 10.1186/s13046-024-03200-x. J Exp Clin Cancer Res. 2024. PMID: 39363363 Free PMC article.
-
RNA Seq profiling reveals a novel expression pattern of TGF-β target genes in human blood eosinophils.Immunol Lett. 2015 Sep;167(1):1-10. doi: 10.1016/j.imlet.2015.06.012. Epub 2015 Jun 22. Immunol Lett. 2015. PMID: 26112417 Free PMC article.
-
The role and mechanisms of macrophage polarization and hepatocyte pyroptosis in acute liver failure.Front Immunol. 2023 Oct 26;14:1279264. doi: 10.3389/fimmu.2023.1279264. eCollection 2023. Front Immunol. 2023. PMID: 37954583 Free PMC article. Review.
-
TGF-β: Many Paths to CD103+ CD8 T Cell Residency.Cells. 2021 Apr 23;10(5):989. doi: 10.3390/cells10050989. Cells. 2021. PMID: 33922441 Free PMC article. Review.
References
-
- Gleizes PE, Munger JS, Nunes I, Harpel JG, Mazzieri R, et al. TGF-β latency: biological significance and mechanisms of activation. Stem Cells. 1997;15:190–97. - PubMed
-
- Munger JS, Harpel JG, Gleizes PE, Mazzieri R, Nunes I, Rifkin DB. Latent transforming growth factor-β: structural features and mechanisms of activation. Kidney Int. 1997;51:1376–82. - PubMed
-
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFβ activation. J Cell Sci. 2003;116:217–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

