Lysophosphatidylcholine triggers TLR2- and TLR4-mediated signaling pathways but counteracts LPS-induced NO synthesis in peritoneal macrophages by inhibiting NF-κB translocation and MAPK/ERK phosphorylation
- PMID: 24312681
- PMCID: PMC3848743
- DOI: 10.1371/journal.pone.0076233
Lysophosphatidylcholine triggers TLR2- and TLR4-mediated signaling pathways but counteracts LPS-induced NO synthesis in peritoneal macrophages by inhibiting NF-κB translocation and MAPK/ERK phosphorylation
Abstract
Background: Lysophosphatidylcholine (LPC) is the main phospholipid component of oxidized low-density lipoprotein (oxLDL) and is usually noted as a marker of several human diseases, such as atherosclerosis, cancer and diabetes. Some studies suggest that oxLDL modulates Toll-like receptor (TLR) signaling. However, effector molecules that are present in oxLDL particles and can trigger TLR signaling are not yet clear. LPC was previously described as an attenuator of sepsis and as an immune suppressor. In the present study, we have evaluated the role of LPC as a dual modulator of the TLR-mediated signaling pathway.
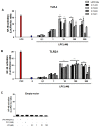
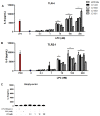
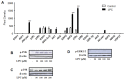
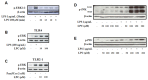
Methodology/principal findings: HEK 293A cells were transfected with TLR expression constructs and stimulated with LPC molecules with different fatty acid chain lengths and saturation levels. All LPC molecules activated both TLR4 and TLR2-1 signaling, as evaluated by NF-қB activation and IL-8 production. These data were confirmed by Western blot analysis of NF-қB translocation in isolated nuclei of peritoneal murine macrophages. However, LPC counteracted the TLR4 signaling induced by LPS. In this case, NF-қB translocation, nitric oxide (NO) synthesis and the expression of inducible nitric oxide synthase (iNOS) were blocked. Moreover, LPC activated the MAP Kinases p38 and JNK, but not ERK, in murine macrophages. Interestingly, LPC blocked LPS-induced ERK activation in peritoneal macrophages but not in TLR-transfected cells.
Conclusions/significance: The above results indicate that LPC is a dual-activity ligand molecule. It is able to trigger a classical proinflammatory phenotype by activating TLR4- and TLR2-1-mediated signaling. However, in the presence of classical TLR ligands, LPC counteracts some of the TLR-mediated intracellular responses, ultimately inducing an anti-inflammatory phenotype; LPC may thus play a role in the regulation of cell immune responses and disease progression.
Conflict of interest statement
Figures

Similar articles
-
Oxidized phospholipid inhibition of toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition.J Biol Chem. 2008 Sep 5;283(36):24748-59. doi: 10.1074/jbc.M800352200. Epub 2008 Jun 17. J Biol Chem. 2008. PMID: 18559343 Free PMC article.
-
Induction of in vitro reprogramming by Toll-like receptor (TLR)2 and TLR4 agonists in murine macrophages: effects of TLR "homotolerance" versus "heterotolerance" on NF-kappa B signaling pathway components.J Immunol. 2003 Jan 1;170(1):508-19. doi: 10.4049/jimmunol.170.1.508. J Immunol. 2003. PMID: 12496438
-
IL-27 promotes nitric oxide production induced by LPS through STAT1, NF-κB and MAPKs.Immunobiology. 2013 Apr;218(4):628-34. doi: 10.1016/j.imbio.2012.07.028. Epub 2012 Aug 8. Immunobiology. 2013. PMID: 22925810
-
Vibrio harveyi infections induce production of proinflammatory cytokines in murine peritoneal macrophages via activation of p38 MAPK and NF-κB pathways, but reversed by PI3K/AKT pathways.Dev Comp Immunol. 2022 Feb;127:104292. doi: 10.1016/j.dci.2021.104292. Epub 2021 Oct 14. Dev Comp Immunol. 2022. PMID: 34656643 Review.
-
Bioactive Compounds and Probiotics Mitigate Mastitis by Targeting NF-κB Signaling Pathway.Biomolecules. 2024 Aug 15;14(8):1011. doi: 10.3390/biom14081011. Biomolecules. 2024. PMID: 39199398 Free PMC article. Review.
Cited by
-
Comprehensive Metabolic Profiling of Inflammation Indicated Key Roles of Glycerophospholipid and Arginine Metabolism in Coronary Artery Disease.Front Immunol. 2022 Mar 8;13:829425. doi: 10.3389/fimmu.2022.829425. eCollection 2022. Front Immunol. 2022. PMID: 35371012 Free PMC article.
-
Lysophosphatidylcholine Induces NLRP3 Inflammasome-Mediated Foam Cell Formation and Pyroptosis in Human Monocytes and Endothelial Cells.Front Immunol. 2020 Jan 9;10:2927. doi: 10.3389/fimmu.2019.02927. eCollection 2019. Front Immunol. 2020. PMID: 31998284 Free PMC article.
-
Recognition of TLR2 N-glycans: critical role in ArtinM immunomodulatory activity.PLoS One. 2014 Jun 3;9(6):e98512. doi: 10.1371/journal.pone.0098512. eCollection 2014. PLoS One. 2014. PMID: 24892697 Free PMC article.
-
Pattern-Recognition Receptors and Immunometabolic Reprogramming: What We Know and What to Explore.J Innate Immun. 2024;16(1):295-323. doi: 10.1159/000539278. Epub 2024 May 13. J Innate Immun. 2024. PMID: 38740018 Free PMC article. Review.
-
Emodin Combined with Nanosilver Inhibited Sepsis by Anti-inflammatory Protection.Front Pharmacol. 2017 Jan 10;7:536. doi: 10.3389/fphar.2016.00536. eCollection 2016. Front Pharmacol. 2017. PMID: 28119611 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

