Cell migration and invasion assays as tools for drug discovery
- PMID: 24310428
- PMCID: PMC3857040
- DOI: 10.3390/pharmaceutics3010107
Cell migration and invasion assays as tools for drug discovery
Abstract
Cell migration and invasion are processes that offer rich targets for intervention in key physiologic and pathologic phenomena such as wound healing and cancer metastasis. With the advent of high-throughput and high content imaging systems, there has been a movement towards the use of physiologically relevant cell-based assays earlier in the testing paradigm. This allows more effective identification of lead compounds and recognition of undesirable effects sooner in the drug discovery screening process. This article will review the effective use of several principle formats for studying cell motility: scratch assays, transmembrane assays, microfluidic devices and cell exclusion zone assays.
Figures
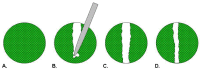
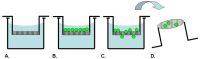
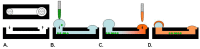
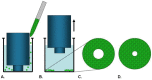
Similar articles
-
Microfluidic systems for high-throughput and high-content screening using the nematode Caenorhabditis elegans.Lab Chip. 2017 Nov 7;17(22):3736-3759. doi: 10.1039/c7lc00509a. Lab Chip. 2017. PMID: 28840220 Review.
-
Current methods for studying metastatic potential of tumor cells.Cancer Cell Int. 2022 Dec 9;22(1):394. doi: 10.1186/s12935-022-02801-w. Cancer Cell Int. 2022. PMID: 36494720 Free PMC article. Review.
-
Real-Time Cell Migration Monitoring to Analyze Drug Synergism in the Scratch Assay Using the IncuCyte System.Methods Mol Biol. 2021;2294:133-142. doi: 10.1007/978-1-0716-1350-4_9. Methods Mol Biol. 2021. PMID: 33742398
-
Microfluidic and Lab-on-a-Chip Systems for Cutaneous Wound Healing Studies.Pharmaceutics. 2021 May 26;13(6):793. doi: 10.3390/pharmaceutics13060793. Pharmaceutics. 2021. PMID: 34073346 Free PMC article. Review.
-
[Methods for studying tumor cell migration and invasiveness].Klin Onkol. 2014;27 Suppl 1:S22-7. doi: 10.14735/amko20141s22. Klin Onkol. 2014. PMID: 24945533 Review. Czech.
Cited by
-
Imidazole Antifungal Drugs Inhibit the Cell Proliferation and Invasion of Human Breast Cancer Cells.Biomol Ther (Seoul). 2018 Sep 1;26(5):494-502. doi: 10.4062/biomolther.2018.042. Biomol Ther (Seoul). 2018. PMID: 30092625 Free PMC article.
-
In vitro wound healing potency of methanolic leaf extract of Aristolochia saccata is possibly mediated by its stimulatory effect on collagen-1 expression.Heliyon. 2019 May 20;5(5):e01648. doi: 10.1016/j.heliyon.2019.e01648. eCollection 2019 May. Heliyon. 2019. PMID: 31193473 Free PMC article.
-
Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress.Redox Biol. 2020 May;32:101500. doi: 10.1016/j.redox.2020.101500. Epub 2020 Mar 11. Redox Biol. 2020. PMID: 32193146 Free PMC article.
-
A Semi-automated and Scalable 3D Spheroid Assay to Study Neuroblast Migration.Stem Cell Reports. 2020 Sep 8;15(3):789-802. doi: 10.1016/j.stemcr.2020.07.012. Epub 2020 Aug 6. Stem Cell Reports. 2020. PMID: 32763162 Free PMC article.
-
Aggregative Perivascular Tumor Cell Growth Pattern of Primary Central Nervous System Lymphomas Is Associated with Hypoxia-Related Endoplasmic Reticulum Stress.J Cancer. 2021 May 5;12(13):3841-3852. doi: 10.7150/jca.54952. eCollection 2021. J Cancer. 2021. PMID: 34093792 Free PMC article.
References
-
- Friedl P., Wolf K. Tumor-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer. 2003;3:362–374. - PubMed
-
- Horwitz R., Webb D. Cell migration. Curr. Biol. 2003;13:R756–R759. - PubMed
-
- Eccles S.A., Box C., Court W. Cell migration/invasion assays and their application in cancer drug discovery. Biotechnol. Ann. Rev. 2005;11:391–421. - PubMed
-
- Barrientos S., Stojadinovic O., Golinko M.S., Brem H., Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Rep. Reg. 2008;16:585–601. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

