Pharmacologic inhibition of vacuolar H+ ATPase reduces physiologic and oncogenic Notch signaling
- PMID: 24309677
- PMCID: PMC5528540
- DOI: 10.1016/j.molonc.2013.11.002
Pharmacologic inhibition of vacuolar H+ ATPase reduces physiologic and oncogenic Notch signaling
Abstract
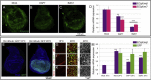
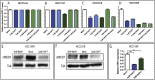
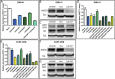
Notch signaling in prominently involved in growth regulation in metazoan tissues. Because of this, Notch is often upregulated in cancer and current efforts point to developing drugs that block its activation. Notch receptor endocytosis towards acidic compartments is a recently appreciated determinant of signaling activation. Vacuolar H(+) ATPase (V-ATPase) is responsible for acidification of endocytic organelles and mutants in V-ATPase subunit encoding genes in model organisms have been recently shown to display loss of Notch signaling. Here, we show that administration of BafilomycinA1 (BafA1), a highly specific V-ATPase inhibitor decreases Notch signaling during Drosophila and Zebrafish development, and in human cells in culture. In normal breast cells, we find that BafA1 treatment leads to accumulation of Notch in the endo-lysosomal system, and reduces its processing and signaling activity. In Notch-addicted breast cancer cells, BafA1 treatment reduces growth in cells expressing membrane tethered forms of Notch, while sparing cells expressing cytoplasmic forms. In contrast, we find that V-ATPase inhibition reduces growth of leukemia cells, without affecting Notch activatory cleavage. However, consistent with the emerging roles of V-ATPase in controlling multiple signaling pathways, in these cells Akt activation is reduced, as it is also the case in BafA1-treated breast cancer cells. Our data support V-ATPase inhibition as a novel therapeutic approach to counteract tumor growth via signaling pathways regulated at the endo-lysosomal level.
Keywords: BafilomycinA1; Cancer; Endocytosis; Notch; V-ATPase.
Copyright © 2013 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Lgl reduces endosomal vesicle acidification and Notch signaling by promoting the interaction between Vap33 and the V-ATPase complex.Sci Signal. 2018 Jun 5;11(533):eaar1976. doi: 10.1126/scisignal.aar1976. Sci Signal. 2018. PMID: 29871910 Free PMC article.
-
The vacuolar ATPase is required for physiological as well as pathological activation of the Notch receptor.Development. 2010 Jun;137(11):1825-32. doi: 10.1242/dev.045484. Development. 2010. PMID: 20460366 Free PMC article.
-
The H(+) vacuolar ATPase maintains neural stem cells in the developing mouse cortex.Stem Cells Dev. 2011 May;20(5):843-50. doi: 10.1089/scd.2010.0484. Epub 2011 Jan 19. Stem Cells Dev. 2011. PMID: 21126173 Free PMC article.
-
The curious case of vacuolar ATPase: regulation of signaling pathways.Mol Cancer. 2018 Feb 15;17(1):41. doi: 10.1186/s12943-018-0811-3. Mol Cancer. 2018. PMID: 29448933 Free PMC article. Review.
-
The emerging roles of vacuolar-type ATPase-dependent Lysosomal acidification in neurodegenerative diseases.Transl Neurodegener. 2020 May 11;9(1):17. doi: 10.1186/s40035-020-00196-0. Transl Neurodegener. 2020. PMID: 32393395 Free PMC article. Review.
Cited by
-
A Review of Notch Processing With New Insights Into Ligand-Independent Notch Signaling in T-Cells.Front Immunol. 2018 Jun 1;9:1230. doi: 10.3389/fimmu.2018.01230. eCollection 2018. Front Immunol. 2018. PMID: 29910816 Free PMC article. Review.
-
Regulation of NOTCH signaling by RAB7 and RAB8 requires carboxyl methylation by ICMT.J Cell Biol. 2017 Dec 4;216(12):4165-4182. doi: 10.1083/jcb.201701053. Epub 2017 Oct 19. J Cell Biol. 2017. PMID: 29051265 Free PMC article.
-
Drug Sequestration in Lysosomes as One of the Mechanisms of Chemoresistance of Cancer Cells and the Possibilities of Its Inhibition.Int J Mol Sci. 2020 Jun 20;21(12):4392. doi: 10.3390/ijms21124392. Int J Mol Sci. 2020. PMID: 32575682 Free PMC article. Review.
-
BLOS2 negatively regulates Notch signaling during neural and hematopoietic stem and progenitor cell development.Elife. 2016 Oct 10;5:e18108. doi: 10.7554/eLife.18108. Elife. 2016. PMID: 27719760 Free PMC article.
-
Proteome and Phosphoproteome Changes Associated with Prognosis in Acute Myeloid Leukemia.Cancers (Basel). 2020 Mar 17;12(3):709. doi: 10.3390/cancers12030709. Cancers (Basel). 2020. PMID: 32192169 Free PMC article.
References
-
- Agrawal, N. , Frederick, M.J. , Pickering, C.R. , Bettegowda, C. , Chang, K. , Li, R.J. , Fakhry, C. , Xie, T.-X. , Zhang, J. , Wang, J. , Zhang, N. , El-Naggar, A.K. , Jasser, S.A. , Weinstein, J.N. , Treviño, L. , Drummond, J.A. , Muzny, D.M. , Wu, Y. , Wood, L.D. , Hruban, R.H. , Westra, W.H. , Koch, W.M. , Califano, J.A. , Gibbs, R.A. , Sidransky, D. , Vogelstein, B. , Velculescu, V.E. , Papadopoulos, N. , Wheeler, D.A. , Kinzler, K.W. , Myers, J.N. , 2011. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 333, 1154–1157. - PMC - PubMed
-
- Aste-Amezaga, M. , Zhang, N. , Lineberger, J.E. , Arnold, B.A. , Toner, T.J. , Gu, M. , Huang, L. , Vitelli, S. , Vo, K.T. , Haytko, P. , Zhao, J.Z. , Baleydier, F. , L'Heureux, S. , Wang, H. , Gordon, W.R. , Thoryk, E. , Andrawes, M.B. , Tiyanont, K. , Stegmaier, K. , Roti, G. , Ross, K.N. , Franlin, L.L. , Wang, F. , Chastain, M. , Bett, A.J. , Audoly, L.P. , Aster, J.C. , Blacklow, S.C. , Huber, H.E. , 2010. Characterization of Notch1 antibodies that inhibit signaling of both normal and mutated Notch1 receptors. PLoS One. 5, e9094 - PMC - PubMed
-
- Ayyanan, A. , Civenni, G. , Ciarloni, L. , Morel, C. , Mueller, N. , Lefort, K. , Mandinova, A. , Raffoul, W. , Fiche, M. , Dotto, G.P. , Brisken, C. , 2006. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc. Natl. Acad. Sci. U. S. A.. 103, 3799–3804. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

