Structure of the TRPV1 ion channel determined by electron cryo-microscopy
- PMID: 24305160
- PMCID: PMC4078027
- DOI: 10.1038/nature12822
Structure of the TRPV1 ion channel determined by electron cryo-microscopy
Abstract
Transient receptor potential (TRP) channels are sensors for a wide range of cellular and environmental signals, but elucidating how these channels respond to physical and chemical stimuli has been hampered by a lack of detailed structural information. Here we exploit advances in electron cryo-microscopy to determine the structure of a mammalian TRP channel, TRPV1, at 3.4 Å resolution, breaking the side-chain resolution barrier for membrane proteins without crystallization. Like voltage-gated channels, TRPV1 exhibits four-fold symmetry around a central ion pathway formed by transmembrane segments 5-6 (S5-S6) and the intervening pore loop, which is flanked by S1-S4 voltage-sensor-like domains. TRPV1 has a wide extracellular 'mouth' with a short selectivity filter. The conserved 'TRP domain' interacts with the S4-S5 linker, consistent with its contribution to allosteric modulation. Subunit organization is facilitated by interactions among cytoplasmic domains, including amino-terminal ankyrin repeats. These observations provide a structural blueprint for understanding unique aspects of TRP channel function.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


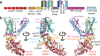
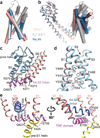
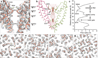
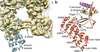


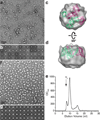


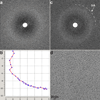

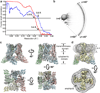
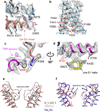
Comment in
-
Structural biology: Ion channel seen by electron microscopy.Nature. 2013 Dec 5;504(7478):93-4. doi: 10.1038/504093a. Nature. 2013. PMID: 24305155 No abstract available.
Similar articles
-
Determining the Crystal Structure of TRPV6.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. PMID: 30299652 Free Books & Documents. Review.
-
The region adjacent to the C-end of the inner gate in transient receptor potential melastatin 8 (TRPM8) channels plays a central role in allosteric channel activation.J Biol Chem. 2014 Oct 10;289(41):28579-94. doi: 10.1074/jbc.M114.577478. Epub 2014 Aug 25. J Biol Chem. 2014. PMID: 25157108 Free PMC article.
-
Cryo-electron microscopy structure of the TRPV2 ion channel.Nat Struct Mol Biol. 2016 Feb;23(2):180-186. doi: 10.1038/nsmb.3159. Epub 2016 Jan 18. Nat Struct Mol Biol. 2016. PMID: 26779611 Free PMC article.
-
Structure of TRPV1 channel revealed by electron cryomicroscopy.Proc Natl Acad Sci U S A. 2008 May 27;105(21):7451-5. doi: 10.1073/pnas.0711835105. Epub 2008 May 19. Proc Natl Acad Sci U S A. 2008. PMID: 18490661 Free PMC article.
-
TRP Channels: What Do They Look Like?In: Emir TLR, editor. Neurobiology of TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. Chapter 1. In: Emir TLR, editor. Neurobiology of TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. Chapter 1. PMID: 29356490 Free Books & Documents. Review.
Cited by
-
Non-averaged single-molecule tertiary structures reveal RNA self-folding through individual-particle cryo-electron tomography.Nat Commun. 2024 Oct 21;15(1):9084. doi: 10.1038/s41467-024-52914-1. Nat Commun. 2024. PMID: 39433544 Free PMC article.
-
Surfactant cocamide monoethanolamide causes eye irritation by activating nociceptor TRPV1 channels.Br J Pharmacol. 2021 Sep;178(17):3448-3462. doi: 10.1111/bph.15491. Epub 2021 Jun 1. Br J Pharmacol. 2021. PMID: 33837959 Free PMC article.
-
Modeling Suggests TRPC3 Hydrogen Bonding and Not Phosphorylation Contributes to the Ataxia Phenotype of the Moonwalker Mouse.Biochemistry. 2015 Jul 7;54(26):4033-41. doi: 10.1021/acs.biochem.5b00235. Epub 2015 Jun 26. Biochemistry. 2015. PMID: 26112884 Free PMC article.
-
A journey from molecule to physiology and in silico tools for drug discovery targeting the transient receptor potential vanilloid type 1 (TRPV1) channel.Front Pharmacol. 2024 Jan 24;14:1251061. doi: 10.3389/fphar.2023.1251061. eCollection 2023. Front Pharmacol. 2024. PMID: 38328578 Free PMC article. Review.
-
Energetics of Ion Permeation in an Open-Activated TRPV1 Channel.Biophys J. 2016 Sep 20;111(6):1214-1222. doi: 10.1016/j.bpj.2016.08.009. Biophys J. 2016. PMID: 27653480 Free PMC article.
References
-
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. - PubMed
-
- Nilius B, Owsianik G. Transient receptor potential channelopathies. Pflugers Arch. 2010;460:437–450. - PubMed
-
- Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

