An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy
- PMID: 24298022
- PMCID: PMC3911487
- DOI: 10.1128/MCB.01307-13
An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy
Abstract
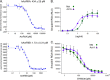
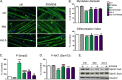
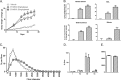
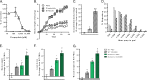
The myostatin/activin type II receptor (ActRII) pathway has been identified to be critical in regulating skeletal muscle size. Several other ligands, including GDF11 and the activins, signal through this pathway, suggesting that the ActRII receptors are major regulatory nodes in the regulation of muscle mass. We have developed a novel, human anti-ActRII antibody (bimagrumab, or BYM338) to prevent binding of ligands to the receptors and thus inhibit downstream signaling. BYM338 enhances differentiation of primary human skeletal myoblasts and counteracts the inhibition of differentiation induced by myostatin or activin A. BYM338 prevents myostatin- or activin A-induced atrophy through inhibition of Smad2/3 phosphorylation, thus sparing the myosin heavy chain from degradation. BYM338 dramatically increases skeletal muscle mass in mice, beyond sole inhibition of myostatin, detected by comparing the antibody with a myostatin inhibitor. A mouse version of the antibody induces enhanced muscle hypertrophy in myostatin mutant mice, further confirming a beneficial effect on muscle growth beyond myostatin inhibition alone through blockade of ActRII ligands. BYM338 protects muscles from glucocorticoid-induced atrophy and weakness via prevention of muscle and tetanic force losses. These data highlight the compelling therapeutic potential of BYM338 for the treatment of skeletal muscle atrophy and weakness in multiple settings.
Figures

Similar articles
-
Blockade of activin type II receptors with a dual anti-ActRIIA/IIB antibody is critical to promote maximal skeletal muscle hypertrophy.Proc Natl Acad Sci U S A. 2017 Nov 21;114(47):12448-12453. doi: 10.1073/pnas.1707925114. Epub 2017 Nov 6. Proc Natl Acad Sci U S A. 2017. PMID: 29109273 Free PMC article.
-
Antibody blockade of activin type II receptors preserves skeletal muscle mass and enhances fat loss during GLP-1 receptor agonism.Mol Metab. 2024 Feb;80:101880. doi: 10.1016/j.molmet.2024.101880. Epub 2024 Jan 11. Mol Metab. 2024. PMID: 38218536 Free PMC article.
-
Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size.Am J Physiol Cell Physiol. 2009 Jun;296(6):C1258-70. doi: 10.1152/ajpcell.00105.2009. Epub 2009 Apr 8. Am J Physiol Cell Physiol. 2009. PMID: 19357233
-
PI3 kinase regulation of skeletal muscle hypertrophy and atrophy.Curr Top Microbiol Immunol. 2010;346:267-78. doi: 10.1007/82_2010_78. Curr Top Microbiol Immunol. 2010. PMID: 20593312 Review.
-
Myostatin/activin pathway antagonism: molecular basis and therapeutic potential.Int J Biochem Cell Biol. 2013 Oct;45(10):2333-47. doi: 10.1016/j.biocel.2013.05.019. Epub 2013 May 28. Int J Biochem Cell Biol. 2013. PMID: 23721881 Review.
Cited by
-
Comparative Analysis of the Extracellular Matrix Proteome across the Myotendinous Junction.J Proteome Res. 2020 Oct 2;19(10):3955-3967. doi: 10.1021/acs.jproteome.0c00248. Epub 2020 Sep 14. J Proteome Res. 2020. PMID: 32830507 Free PMC article.
-
The Current Landscape of Pharmacotherapies for Sarcopenia.Drugs Aging. 2024 Feb;41(2):83-112. doi: 10.1007/s40266-023-01093-7. Epub 2024 Feb 5. Drugs Aging. 2024. PMID: 38315328
-
Low Myostatin Serum Levels Are Associated with Poor Outcome in Critically Ill Patients.Diagnostics (Basel). 2020 Aug 8;10(8):574. doi: 10.3390/diagnostics10080574. Diagnostics (Basel). 2020. PMID: 32784522 Free PMC article.
-
Identification of Molecules from Coffee Silverskin That Suppresses Myostatin Activity and Improves Muscle Mass and Strength in Mice.Molecules. 2021 May 3;26(9):2676. doi: 10.3390/molecules26092676. Molecules. 2021. PMID: 34063650 Free PMC article.
-
Activin A Causes Muscle Atrophy through MEF2C-Dependent Impaired Myogenesis.Cells. 2022 Mar 25;11(7):1119. doi: 10.3390/cells11071119. Cells. 2022. PMID: 35406681 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
