Pentameric complex of viral glycoprotein H is the primary target for potent neutralization by a human cytomegalovirus vaccine
- PMID: 24297878
- PMCID: PMC3870741
- DOI: 10.1073/pnas.1316517110
Pentameric complex of viral glycoprotein H is the primary target for potent neutralization by a human cytomegalovirus vaccine
Abstract
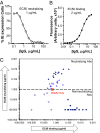
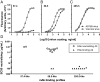
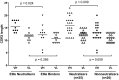
Human cytomegalovirus (HCMV) can cause serious morbidity/mortality in transplant patients, and congenital HCMV infection can lead to birth defects. Developing an effective HCMV vaccine is a high medical priority. One of the challenges to the efforts has been our limited understanding of the viral antigens important for protective antibodies. Receptor-mediated viral entry to endothelial/epithelial cells requires a glycoprotein H (gH) complex comprising five viral proteins (gH, gL, UL128, UL130, and UL131). This gH complex is notably missing from HCMV laboratory strains as well as HCMV vaccines previously evaluated in the clinic. To support a unique vaccine concept based on the pentameric gH complex, we established a panel of 45 monoclonal antibodies (mAbs) from a rabbit immunized with an experimental vaccine virus in which the expression of the pentameric gH complex was restored. Over one-half (25 of 45) of the mAbs have neutralizing activity. Interestingly, affinity for an antibody to bind virions was not correlated with its ability to neutralize the virus. Genetic analysis of the 45 mAbs based on their heavy- and light-chain sequences identified at least 26 B-cell linage groups characterized by distinct binding or neutralizing properties. Moreover, neutralizing antibodies possessed longer complementarity-determining region 3 for both heavy and light chains than those with no neutralizing activity. Importantly, potent neutralizing mAbs reacted to the pentameric gH complex but not to gB. Thus, the pentameric gH complex is the primary target for antiviral antibodies by vaccination.
Keywords: antiviral antibody; monoclonal antibody.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Human cytomegalovirus vaccine based on the envelope gH/gL pentamer complex.PLoS Pathog. 2014 Nov 20;10(11):e1004524. doi: 10.1371/journal.ppat.1004524. eCollection 2014 Nov. PLoS Pathog. 2014. PMID: 25412505 Free PMC article.
-
Human cytomegalovirus gH/gL/UL128/UL130/UL131A complex elicits potently neutralizing antibodies in mice.Vaccine. 2014 Jun 24;32(30):3796-804. doi: 10.1016/j.vaccine.2014.05.004. Epub 2014 May 14. Vaccine. 2014. PMID: 24837507
-
Dense Bodies of a gH/gL/UL128/UL130/UL131 Pentamer-Repaired Towne Strain of Human Cytomegalovirus Induce an Enhanced Neutralizing Antibody Response.J Virol. 2019 Aug 13;93(17):e00931-19. doi: 10.1128/JVI.00931-19. Print 2019 Sep 1. J Virol. 2019. PMID: 31189713 Free PMC article.
-
Exploiting viral natural history for vaccine development.Med Microbiol Immunol. 2015 Jun;204(3):255-62. doi: 10.1007/s00430-015-0406-1. Epub 2015 Mar 21. Med Microbiol Immunol. 2015. PMID: 25794555 Free PMC article. Review.
-
Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism.Viruses. 2018 Dec 11;10(12):704. doi: 10.3390/v10120704. Viruses. 2018. PMID: 30544948 Free PMC article. Review.
Cited by
-
Convalescent Immunity to Guinea Pig Cytomegalovirus Induces Limited Cross Strain Protection against Re-Infection but High-Level Protection against Congenital Disease.Int J Mol Sci. 2020 Aug 20;21(17):5997. doi: 10.3390/ijms21175997. Int J Mol Sci. 2020. PMID: 32825429 Free PMC article.
-
Genome Sequence of Human Cytomegalovirus Ig-KG-H2, a Variant of Strain KG Propagated in the Presence of Neutralizing Antibodies.Microbiol Resour Announc. 2020 Apr 23;9(17):e00063-20. doi: 10.1128/MRA.00063-20. Microbiol Resour Announc. 2020. PMID: 32327516 Free PMC article.
-
Human Cytomegalovirus Particles Treated with Specific Antibodies Induce Intrinsic and Adaptive but Not Innate Immune Responses.J Virol. 2017 Oct 27;91(22):e00678-17. doi: 10.1128/JVI.00678-17. Print 2017 Nov 15. J Virol. 2017. PMID: 28878085 Free PMC article.
-
Active evolution of memory B-cells specific to viral gH/gL/pUL128/130/131 pentameric complex in healthy subjects with silent human cytomegalovirus infection.Oncotarget. 2017 Jun 3;8(43):73654-73669. doi: 10.18632/oncotarget.18359. eCollection 2017 Sep 26. Oncotarget. 2017. PMID: 29088734 Free PMC article.
-
Targeting Human-Cytomegalovirus-Infected Cells by Redirecting T Cells Using an Anti-CD3/Anti-Glycoprotein B Bispecific Antibody.Antimicrob Agents Chemother. 2017 Dec 21;62(1):e01719-17. doi: 10.1128/AAC.01719-17. Print 2018 Jan. Antimicrob Agents Chemother. 2017. PMID: 29038280 Free PMC article.
References
-
- Beam E, Razonable RR. Cytomegalovirus in solid organ transplantation: Epidemiology, prevention, and treatment. Curr Infect Dis Rep. 2012;14(6):633–641. - PubMed
-
- Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601–2614. - PubMed
-
- Kotton CN. Management of cytomegalovirus infection in solid organ transplantation. Nat Rev Nephrol. 2010;6(12):711–721. - PubMed
-
- Opelz G, Dohler B, Ruhenstroth A. Cytomegalovirus prophylaxis and graft outcome in solid organ transplantation: A collaborative transplant study report. Am J Transplant. 2004;4(6):928–936. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

