Effects of cochlear ablation on amino acid levels in the rat cochlear nucleus and superior olive
- PMID: 24291808
- PMCID: PMC5819880
- DOI: 10.1016/j.heares.2013.11.005
Effects of cochlear ablation on amino acid levels in the rat cochlear nucleus and superior olive
Abstract
Amino acids have important roles in the chemistry of the auditory system, including communication among neurons. There is much evidence for glutamate as a neurotransmitter from auditory nerve fibers to cochlear nucleus neurons. Previous studies in rodents have examined effects of removal of auditory nerve input by cochlear ablation on levels, uptake and release of glutamate in cochlear nucleus subdivisions, as well as on glutamate receptors. Effects have also been reported on uptake and release of γ-aminobutyrate (GABA) and glycine, two other amino acids strongly implicated in cochlear nucleus synaptic transmission. We mapped the effects of cochlear ablation on the levels of amino acids, including glutamate, GABA, glycine, aspartate, glutamine, taurine, serine, threonine, and arginine, in microscopic subregions of the rat cochlear nucleus. Submicrogram-size samples microdissected from freeze-dried brainstem sections were assayed for amino acid levels by high performance liquid chromatography. After cochlear ablation, glutamate and aspartate levels decreased by 2 days in regions receiving relatively dense innervation from the auditory nerve, whereas the levels of most other amino acids increased. The results are consistent with a close association of glutamate and aspartate with auditory nerve fibers and of other amino acids with other neurons and glia in the cochlear nucleus. A consistent decrease of GABA level in the lateral superior olive could be consistent with a role in some lateral olivocochlear neurons. The results are compared with those obtained with the same methods for the rat vestibular nerve root and nuclei after vestibular ganglionectomy.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures
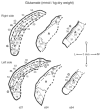
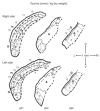
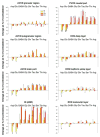
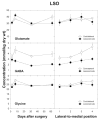

Similar articles
-
Effects of brainstem lesions on amino acid levels in the rat cochlear nucleus.Hear Res. 2021 Apr;403:108187. doi: 10.1016/j.heares.2021.108187. Epub 2021 Jan 29. Hear Res. 2021. PMID: 33578260 Free PMC article.
-
Amino acid concentrations in rat cochlear nucleus and superior olive.Hear Res. 2000 Dec;150(1-2):189-205. doi: 10.1016/s0378-5955(00)00199-4. Hear Res. 2000. PMID: 11077203
-
Cochlear ablation effects on amino acid levels in the chinchilla cochlear nucleus.Neuroscience. 2015 Jun 25;297:137-59. doi: 10.1016/j.neuroscience.2015.03.055. Epub 2015 Apr 1. Neuroscience. 2015. PMID: 25839146
-
The multiple functions of T stellate/multipolar/chopper cells in the ventral cochlear nucleus.Hear Res. 2011 Jun;276(1-2):61-9. doi: 10.1016/j.heares.2010.10.018. Epub 2010 Nov 4. Hear Res. 2011. PMID: 21056098 Free PMC article. Review.
-
Diversity and plasticity in amino acid receptor subunits in the rat auditory brain stem.Hear Res. 2000 Sep;147(1-2):137-44. doi: 10.1016/s0378-5955(00)00127-1. Hear Res. 2000. PMID: 10962180 Review.
Cited by
-
Metabolic Abnormalities Linked to Auditory Pathways in ApoE-Knockout HEI-OC1 Cells: A Transcription-Metabolism Co-Analysis.Biomolecules. 2022 Sep 1;12(9):1217. doi: 10.3390/biom12091217. Biomolecules. 2022. PMID: 36139057 Free PMC article.
-
Effects of brainstem lesions on amino acid levels in the rat cochlear nucleus.Hear Res. 2021 Apr;403:108187. doi: 10.1016/j.heares.2021.108187. Epub 2021 Jan 29. Hear Res. 2021. PMID: 33578260 Free PMC article.
-
Central gain control in tinnitus and hyperacusis.Front Neurol. 2014 Oct 24;5:206. doi: 10.3389/fneur.2014.00206. eCollection 2014. Front Neurol. 2014. PMID: 25386157 Free PMC article. Review.
-
Insult-induced adaptive plasticity of the auditory system.Front Neurosci. 2014 May 23;8:110. doi: 10.3389/fnins.2014.00110. eCollection 2014. Front Neurosci. 2014. PMID: 24904256 Free PMC article. Review.
-
Central plasticity and dysfunction elicited by aural deprivation in the critical period.Front Neural Circuits. 2015 Jun 2;9:26. doi: 10.3389/fncir.2015.00026. eCollection 2015. Front Neural Circuits. 2015. PMID: 26082685 Free PMC article. Review.
References
-
- Abbott SD, Hughes LF, Bauer CA, Salvi R, Caspary DM. Detection of glutamate decarboxylase isoforms in rat inferior colliculus following acoustic exposure. Neuroscience. 1999;93:1375–1381. - PubMed
-
- Albrecht J, Schousboe A. Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem Res. 2005;30:1615–1621. - PubMed
-
- Aldskogius H, Kozlova EN. Central neuron-glial and glial-glial interactions following axon injury. Prog Neurobiol. 1998;55:1–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

