megaTALs: a rare-cleaving nuclease architecture for therapeutic genome engineering
- PMID: 24285304
- PMCID: PMC3936731
- DOI: 10.1093/nar/gkt1224
megaTALs: a rare-cleaving nuclease architecture for therapeutic genome engineering
Abstract
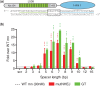
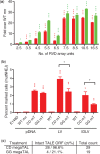
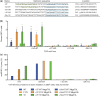
Rare-cleaving endonucleases have emerged as important tools for making targeted genome modifications. While multiple platforms are now available to generate reagents for research applications, each existing platform has significant limitations in one or more of three key properties necessary for therapeutic application: efficiency of cleavage at the desired target site, specificity of cleavage (i.e. rate of cleavage at 'off-target' sites), and efficient/facile means for delivery to desired target cells. Here, we describe the development of a single-chain rare-cleaving nuclease architecture, which we designate 'megaTAL', in which the DNA binding region of a transcription activator-like (TAL) effector is used to 'address' a site-specific meganuclease adjacent to a single desired genomic target site. This architecture allows the generation of extremely active and hyper-specific compact nucleases that are compatible with all current viral and nonviral cell delivery methods.
Figures


Similar articles
-
Assembly and characterization of megaTALs for hyperspecific genome engineering applications.Methods Mol Biol. 2015;1239:171-96. doi: 10.1007/978-1-4939-1862-1_9. Methods Mol Biol. 2015. PMID: 25408406
-
MegaTevs: single-chain dual nucleases for efficient gene disruption.Nucleic Acids Res. 2014 Jul;42(13):8816-29. doi: 10.1093/nar/gku573. Epub 2014 Jul 10. Nucleic Acids Res. 2014. PMID: 25013171 Free PMC article.
-
Progressive engineering of a homing endonuclease genome editing reagent for the murine X-linked immunodeficiency locus.Nucleic Acids Res. 2014 Jun;42(10):6463-75. doi: 10.1093/nar/gku224. Epub 2014 Mar 25. Nucleic Acids Res. 2014. PMID: 24682825 Free PMC article.
-
Nuclease-mediated genome editing: At the front-line of functional genomics technology.Dev Growth Differ. 2014 Jan;56(1):2-13. doi: 10.1111/dgd.12111. Epub 2014 Jan 5. Dev Growth Differ. 2014. PMID: 24387662 Review.
-
Recent progress in genome engineering techniques in the silkworm, Bombyx mori.Dev Growth Differ. 2014 Jan;56(1):14-25. doi: 10.1111/dgd.12096. Epub 2013 Nov 1. Dev Growth Differ. 2014. PMID: 24175911 Review.
Cited by
-
Adenovirus vectors in hematopoietic stem cell genome editing.FEBS Lett. 2019 Dec;593(24):3623-3648. doi: 10.1002/1873-3468.13668. Epub 2019 Nov 20. FEBS Lett. 2019. PMID: 31705806 Free PMC article. Review.
-
Evaluation of TCR Gene Editing Achieved by TALENs, CRISPR/Cas9, and megaTAL Nucleases.Mol Ther. 2016 Mar;24(3):570-81. doi: 10.1038/mt.2015.197. Epub 2015 Oct 27. Mol Ther. 2016. PMID: 26502778 Free PMC article.
-
Targeted gene knock-in by homology-directed genome editing using Cas9 ribonucleoprotein and AAV donor delivery.Nucleic Acids Res. 2017 Jun 20;45(11):e98. doi: 10.1093/nar/gkx154. Nucleic Acids Res. 2017. PMID: 28334779 Free PMC article.
-
Optimized tuning of TALEN specificity using non-conventional RVDs.Sci Rep. 2015 Jan 30;5:8150. doi: 10.1038/srep08150. Sci Rep. 2015. PMID: 25632877 Free PMC article.
-
Customizing the genome as therapy for the β-hemoglobinopathies.Blood. 2016 May 26;127(21):2536-45. doi: 10.1182/blood-2016-01-678128. Epub 2016 Apr 6. Blood. 2016. PMID: 27053533 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

