The emerging role of the phosphatidylinositol 3-kinase/ akt/mammalian target of rapamycin signaling network in cancer stem cell biology
- PMID: 24281174
- PMCID: PMC3837323
- DOI: 10.3390/cancers2031576
The emerging role of the phosphatidylinositol 3-kinase/ akt/mammalian target of rapamycin signaling network in cancer stem cell biology
Abstract
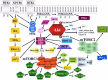
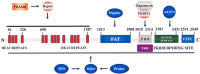
The cancer stem cell theory entails the existence of a hierarchically organized, rare population of cells which are responsible for tumor initiation, self-renewal/maintenance, and mutation accumulation. The cancer stem cell proposition could explain the high frequency of cancer relapse and resistance to currently available therapies. The phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathway regulates a wide array of physiological cell functions which include differentiation, proliferation, survival, metabolism, autophagy, and motility. Dysregulated PI3K/Akt/mTOR signaling has been documented in many types of neoplasias. It is now emerging that this signaling network plays a key role in cancer stem cell biology. Interestingly, cancer stem cells displayed preferential sensitivity to pathway inhibition when compared to healthy stem cells. This observation provides the proof-of-principle that functional differences in signaling pathways between neoplastic stem cells and healthy stem cells could be identified. In this review, we present the evidence which links the signals emanating from the PI3K/Akt/mTOR cascade with the functions of cancer stem cells, both in solid and hematological tumors. We then highlight how targeting PI3K/Akt/mTOR signaling with small molecules could improve cancer patient outcome.
Figures
Similar articles
-
Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling network in cancer stem cells.Curr Med Chem. 2011;18(18):2715-26. doi: 10.2174/092986711796011201. Curr Med Chem. 2011. PMID: 21649579 Review.
-
The emerging role of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling network in normal myelopoiesis and leukemogenesis.Biochim Biophys Acta. 2010 Sep;1803(9):991-1002. doi: 10.1016/j.bbamcr.2010.04.005. Epub 2010 Apr 23. Biochim Biophys Acta. 2010. PMID: 20399811 Review.
-
The phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin signaling network and the control of normal myelopoiesis.Histol Histopathol. 2010 May;25(5):669-80. doi: 10.14670/HH-25.669. Histol Histopathol. 2010. PMID: 20238304 Review.
-
Two hits are better than one: targeting both phosphatidylinositol 3-kinase and mammalian target of rapamycin as a therapeutic strategy for acute leukemia treatment.Oncotarget. 2012 Apr;3(4):371-94. doi: 10.18632/oncotarget.477. Oncotarget. 2012. PMID: 22564882 Free PMC article. Review.
-
Dual Inhibition of Phosphatidylinositol 3-Kinase and Mammalian Target of Rapamycin: a Therapeutic Strategy for Acute Leukemias.Curr Cancer Drug Targets. 2012 Nov 21. Online ahead of print. Curr Cancer Drug Targets. 2012. PMID: 23215723
Cited by
-
Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health.Oncotarget. 2011 Mar;2(3):135-64. doi: 10.18632/oncotarget.240. Oncotarget. 2011. PMID: 21411864 Free PMC article. Review.
-
A two-stage genome-wide association study identifies novel germline genetic variations in CACNA2D3 associated with radiotherapy response in nasopharyngeal carcinoma.J Transl Med. 2023 Jan 9;21(1):11. doi: 10.1186/s12967-022-03819-4. J Transl Med. 2023. PMID: 36624463 Free PMC article.
-
Cancer stem cells in haematological malignancies.Contemp Oncol (Pozn). 2015;19(1A):A1-6. doi: 10.5114/wo.2014.47127. Contemp Oncol (Pozn). 2015. PMID: 25691816 Free PMC article. Review.
-
A first-in-human phase I study of TAS-117, an allosteric AKT inhibitor, in patients with advanced solid tumors.Cancer Chemother Pharmacol. 2024 Jun;93(6):605-616. doi: 10.1007/s00280-023-04631-7. Epub 2024 Feb 27. Cancer Chemother Pharmacol. 2024. PMID: 38411735 Free PMC article. Clinical Trial.
-
Arsenic and benzo[a]pyrene co-exposure acts synergistically in inducing cancer stem cell-like property and tumorigenesis by epigenetically down-regulating SOCS3 expression.Environ Int. 2020 Apr;137:105560. doi: 10.1016/j.envint.2020.105560. Epub 2020 Feb 18. Environ Int. 2020. PMID: 32062438 Free PMC article.
References
-
- Misaghian N., Ligresti G., Steelman L.S., Bertrand F.E., Basecke J., Libra M., Nicoletti F., Stivala F., Milella M., Tafuri A., Cervello M., Martelli A.M., McCubrey J.A. Targeting the leukemic stem cell: The Holy Grail of leukemia therapy. Leukemia. 2009;23:25–42. doi: 10.1038/leu.2008.246. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

