Lung collagens perpetuate pulmonary fibrosis via CD204 and M2 macrophage activation
- PMID: 24278429
- PMCID: PMC3835428
- DOI: 10.1371/journal.pone.0081382
Lung collagens perpetuate pulmonary fibrosis via CD204 and M2 macrophage activation
Abstract
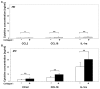
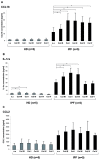
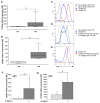
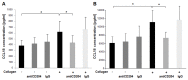
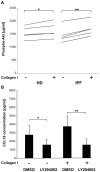
Idiopathic pulmonary fibrosis is characterized by abundant collagen production and accumulation of alternatively activated macrophages (M2) in the lower respiratory tract. Mechanisms as to how alveolar macrophages are activated by collagen breakdown products are unknown. Alveolar macrophages were obtained by bronchoalveolar lavage from 30 patients with idiopathic pulmonary fibrosis (IPF) and 37 healthy donors (HD). Alveolar macrophages were cultured in the presence of collagen type I, III, IV and V monomers w/wo a neutralizing antibody against scavenger receptor I class A (CD204). Culture supernatants were assayed for the M2 markers CCL18, CCL2, and interleukin-1 receptor antagonist (IL-1ra) by ELISA. Furthermore, expression of phospho-Akt was measured using ELISA and expression of CD204 by RT-PCR and flow cytometry. Stimulation with collagen type I and III monomers significantly up-regulated CCL18, IL-1ra production of alveolar macrophages. Furthermore, expression of CCL2 and CD204 were up-regulated by collagen type I exposure. In addition, collagen type I stimulation increased pospho-Akt expression. Collagen type I effects were abrogated by neutralizing antiCD204 and a non-selective Phosphatidylinositide 3-kinase inhibitor (LY294002). Spontaneous CD204 expression of alveolar macrophages was significantly increased in patients with IPF. In conclusion, our findings demonstrate that monomeric collagen type I via CD204 induces phospho-Akt expression shifting alveolar macrophages to the profibrotic M2 type. Innate immune responses induced by collagen monomers might perpetuate pulmonary fibrosis.
Conflict of interest statement
Figures
Similar articles
-
A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18.Am J Respir Crit Care Med. 2006 Apr 1;173(7):781-92. doi: 10.1164/rccm.200509-1518OC. Epub 2006 Jan 13. Am J Respir Crit Care Med. 2006. PMID: 16415274
-
Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction.Clin Immunol. 2010 Oct;137(1):89-101. doi: 10.1016/j.clim.2010.06.017. Epub 2010 Jul 31. Clin Immunol. 2010. PMID: 20674506
-
IL20Rb aggravates pulmonary fibrosis through enhancing bone marrow derived profibrotic macrophage activation.Pharmacol Res. 2024 May;203:107178. doi: 10.1016/j.phrs.2024.107178. Epub 2024 Apr 5. Pharmacol Res. 2024. PMID: 38583686
-
Macrophage polarization and its impact on idiopathic pulmonary fibrosis.Front Immunol. 2024 Jul 26;15:1444964. doi: 10.3389/fimmu.2024.1444964. eCollection 2024. Front Immunol. 2024. PMID: 39131154 Free PMC article. Review.
-
Regulation of alveolar macrophage death in pulmonary fibrosis: a review.Apoptosis. 2023 Dec;28(11-12):1505-1519. doi: 10.1007/s10495-023-01888-4. Epub 2023 Sep 14. Apoptosis. 2023. PMID: 37707713 Free PMC article. Review.
Cited by
-
Alteration of the Antitumor Immune Response by Cancer-Associated Fibroblasts.Front Immunol. 2018 Mar 1;9:414. doi: 10.3389/fimmu.2018.00414. eCollection 2018. Front Immunol. 2018. PMID: 29545811 Free PMC article. Review.
-
Cancer-Associated Fibroblasts: Accomplices in the Tumor Immune Evasion.Cancers (Basel). 2020 Oct 14;12(10):2969. doi: 10.3390/cancers12102969. Cancers (Basel). 2020. PMID: 33066357 Free PMC article. Review.
-
Protective role of spleen-derived macrophages in lung inflammation, injury, and fibrosis induced by nitrogen mustard.Am J Physiol Lung Cell Mol Physiol. 2015 Dec 15;309(12):L1487-98. doi: 10.1152/ajplung.00276.2015. Epub 2015 Oct 16. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 26475734 Free PMC article.
-
Rac2 is required for alternative macrophage activation and bleomycin induced pulmonary fibrosis; a macrophage autonomous phenotype.PLoS One. 2017 Aug 17;12(8):e0182851. doi: 10.1371/journal.pone.0182851. eCollection 2017. PLoS One. 2017. PMID: 28817691 Free PMC article.
-
Macrophages: Key orchestrators of a tumor microenvironment defined by therapeutic resistance.Mol Immunol. 2019 Jun;110:3-12. doi: 10.1016/j.molimm.2017.12.003. Epub 2017 Dec 19. Mol Immunol. 2019. PMID: 29273393 Free PMC article. Review.
References
-
- Hayashi T, Stetler-Stevenson WG, Fleming MV, Fishback N, Koss MN et al. (1996) Immunohistochemical study of metalloproteinases and their tissue inhibitors in the lungs of patients with diffuse alveolar damage and idiopathic pulmonary fibrosis. Am J Pathol 149: 1241-1256. PubMed: 8863673. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

