Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974
- PMID: 24277854
- PMCID: PMC3864356
- DOI: 10.1073/pnas.1314239110
Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974
Abstract
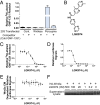
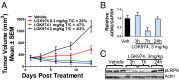
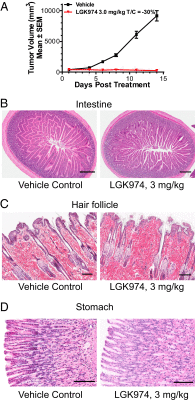
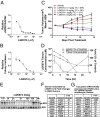
Wnt signaling is one of the key oncogenic pathways in multiple cancers, and targeting this pathway is an attractive therapeutic approach. However, therapeutic success has been limited because of the lack of therapeutic agents for targets in the Wnt pathway and the lack of a defined patient population that would be sensitive to a Wnt inhibitor. We developed a screen for small molecules that block Wnt secretion. This effort led to the discovery of LGK974, a potent and specific small-molecule Porcupine (PORCN) inhibitor. PORCN is a membrane-bound O-acyltransferase that is required for and dedicated to palmitoylation of Wnt ligands, a necessary step in the processing of Wnt ligand secretion. We show that LGK974 potently inhibits Wnt signaling in vitro and in vivo, including reduction of the Wnt-dependent LRP6 phosphorylation and the expression of Wnt target genes, such as AXIN2. LGK974 is potent and efficacious in multiple tumor models at well-tolerated doses in vivo, including murine and rat mechanistic breast cancer models driven by MMTV-Wnt1 and a human head and neck squamous cell carcinoma model (HN30). We also show that head and neck cancer cell lines with loss-of-function mutations in the Notch signaling pathway have a high response rate to LGK974. Together, these findings provide both a strategy and tools for targeting Wnt-driven cancers through the inhibition of PORCN.
Keywords: HNSCC; Wnt inhibition; β-catenin.
Conflict of interest statement
Conflict of interest statement: J. Liu, S.P., M.H.H., N.N., T.W., S.K., D.C., J. Li, C.T., A.P., A.S., C.K., Y.W., C.L., P.M., W.R.S., L.P., A.L.B., H.M.S., M.E.M., J. Che, G.V., and J.L.H. are employees of Novartis.
Figures

Similar articles
-
Porcupine Inhibitor LGK974 Downregulates the Wnt Signaling Pathway and Inhibits Clear Cell Renal Cell Carcinoma.Biomed Res Int. 2020 Feb 13;2020:2527643. doi: 10.1155/2020/2527643. eCollection 2020. Biomed Res Int. 2020. PMID: 32104684 Free PMC article.
-
Porcupine inhibitors: Novel and emerging anti-cancer therapeutics targeting the Wnt signaling pathway.Pharmacol Res. 2021 May;167:105532. doi: 10.1016/j.phrs.2021.105532. Epub 2021 Mar 4. Pharmacol Res. 2021. PMID: 33677106 Review.
-
Porcupine inhibitor LGK‑974 inhibits Wnt/β‑catenin signaling and modifies tumor‑associated macrophages resulting in inhibition of the malignant behaviors of non‑small cell lung cancer cells.Mol Med Rep. 2021 Aug;24(2):550. doi: 10.3892/mmr.2021.12189. Epub 2021 Jun 3. Mol Med Rep. 2021. PMID: 34080032 Free PMC article.
-
Mechanisms and inhibition of Porcupine-mediated Wnt acylation.Nature. 2022 Jul;607(7920):816-822. doi: 10.1038/s41586-022-04952-2. Epub 2022 Jul 13. Nature. 2022. PMID: 35831507 Free PMC article.
-
Chemical Modulation of WNT Signaling in Cancer.Prog Mol Biol Transl Sci. 2018 Jan;153:245-269. doi: 10.1016/bs.pmbts.2017.11.008. Epub 2018 Jan 8. Prog Mol Biol Transl Sci. 2018. PMID: 29389519 Review.
Cited by
-
From Good to Bad: The Opposing Effects of PTHrP on Tumor Growth, Dormancy, and Metastasis Throughout Cancer Progression.Front Oncol. 2021 Mar 22;11:644303. doi: 10.3389/fonc.2021.644303. eCollection 2021. Front Oncol. 2021. PMID: 33828987 Free PMC article. Review.
-
LGR5 Activates Noncanonical Wnt Signaling and Inhibits Aldosterone Production in the Human Adrenal.J Clin Endocrinol Metab. 2015 Jun;100(6):E836-44. doi: 10.1210/jc.2015-1734. Epub 2015 Apr 27. J Clin Endocrinol Metab. 2015. PMID: 25915569 Free PMC article.
-
Phase Ib Study of Wnt Inhibitor Ipafricept with Gemcitabine and nab-paclitaxel in Patients with Previously Untreated Stage IV Pancreatic Cancer.Clin Cancer Res. 2020 Oct 15;26(20):5348-5357. doi: 10.1158/1078-0432.CCR-20-0489. Epub 2020 Jul 21. Clin Cancer Res. 2020. PMID: 32694153 Free PMC article. Clinical Trial.
-
Wnt Lipidation and Modifiers in Intestinal Carcinogenesis and Cancer.Cancers (Basel). 2016 Jul 18;8(7):69. doi: 10.3390/cancers8070069. Cancers (Basel). 2016. PMID: 27438855 Free PMC article. Review.
-
Triple-negative breast cancer: new perspectives for targeted therapies.Onco Targets Ther. 2015 Jan 16;8:177-93. doi: 10.2147/OTT.S67673. eCollection 2015. Onco Targets Ther. 2015. PMID: 25653541 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

