Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer
- PMID: 24277834
- PMCID: PMC3864274
- DOI: 10.1073/pnas.1320318110
Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer
Abstract
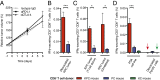
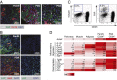
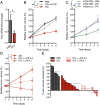
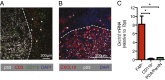
An autochthonous model of pancreatic ductal adenocarcinoma (PDA) permitted the analysis of why immunotherapy is ineffective in this human disease. Despite finding that PDA-bearing mice had cancer cell-specific CD8(+) T cells, the mice, like human patients with PDA, did not respond to two immunological checkpoint antagonists that promote the function of T cells: anti-cytotoxic T-lymphocyte-associated protein 4 (α-CTLA-4) and α-programmed cell death 1 ligand 1 (α-PD-L1). Immune control of PDA growth was achieved, however, by depleting carcinoma-associated fibroblasts (CAFs) that express fibroblast activation protein (FAP). The depletion of the FAP(+) stromal cell also uncovered the antitumor effects of α-CTLA-4 and α-PD-L1, indicating that its immune suppressive activity accounts for the failure of these T-cell checkpoint antagonists. Three findings suggested that chemokine (C-X-C motif) ligand 12 (CXCL12) explained the overriding immunosuppression by the FAP(+) cell: T cells were absent from regions of the tumor containing cancer cells, cancer cells were coated with the chemokine, CXCL12, and the FAP(+) CAF was the principal source of CXCL12 in the tumor. Administering AMD3100, a CXCL12 receptor chemokine (C-X-C motif) receptor 4 inhibitor, induced rapid T-cell accumulation among cancer cells and acted synergistically with α-PD-L1 to greatly diminish cancer cells, which were identified by their loss of heterozygosity of Trp53 gene. The residual tumor was composed only of premalignant epithelial cells and inflammatory cells. Thus, a single protein, CXCL12, from a single stromal cell type, the FAP(+) CAF, may direct tumor immune evasion in a model of human PDA.
Keywords: KPC mouse; T cell exclusion; tumor immunogenicity; tumor stroma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance.Cancer Immunol Res. 2014 Mar;2(3):187-93. doi: 10.1158/2326-6066.CIR-14-0002. Cancer Immunol Res. 2014. PMID: 24778314
-
Targeting CXCR4/CXCL12 axis via [177Lu]Lu-DOTAGA.(SA.FAPi)2 with CXCR4 antagonist in triple-negative breast cancer.Eur J Nucl Med Mol Imaging. 2024 Jul;51(9):2744-2757. doi: 10.1007/s00259-024-06704-y. Epub 2024 Apr 8. Eur J Nucl Med Mol Imaging. 2024. PMID: 38587644 Free PMC article.
-
Blockade of fibroblast activation protein in combination with radiation treatment in murine models of pancreatic adenocarcinoma.PLoS One. 2019 Feb 6;14(2):e0211117. doi: 10.1371/journal.pone.0211117. eCollection 2019. PLoS One. 2019. PMID: 30726287 Free PMC article.
-
The application of the fibroblast activation protein α-targeted immunotherapy strategy.Oncotarget. 2016 May 31;7(22):33472-82. doi: 10.18632/oncotarget.8098. Oncotarget. 2016. PMID: 26985769 Free PMC article. Review.
-
Mechanisms Governing Immunotherapy Resistance in Pancreatic Ductal Adenocarcinoma.Front Immunol. 2021 Jan 28;11:613815. doi: 10.3389/fimmu.2020.613815. eCollection 2020. Front Immunol. 2021. PMID: 33584701 Free PMC article. Review.
Cited by
-
Senescent Tumor Cells Build a Cytokine Shield in Colorectal Cancer.Adv Sci (Weinh). 2021 Jan 4;8(4):2002497. doi: 10.1002/advs.202002497. eCollection 2021 Feb. Adv Sci (Weinh). 2021. PMID: 33643790 Free PMC article.
-
Tumor microenvironment in chemoresistance, metastasis and immunotherapy of pancreatic cancer.Am J Cancer Res. 2020 Jul 1;10(7):1937-1953. eCollection 2020. Am J Cancer Res. 2020. PMID: 32774994 Free PMC article. Review.
-
Organoid models of human and mouse ductal pancreatic cancer.Cell. 2015 Jan 15;160(1-2):324-38. doi: 10.1016/j.cell.2014.12.021. Epub 2014 Dec 31. Cell. 2015. PMID: 25557080 Free PMC article.
-
Genetically Engineered Mouse Models of Pancreatic Cancer: The KPC Model (LSL-Kras(G12D/+) ;LSL-Trp53(R172H/+) ;Pdx-1-Cre), Its Variants, and Their Application in Immuno-oncology Drug Discovery.Curr Protoc Pharmacol. 2016 Jun 1;73:14.39.1-14.39.20. doi: 10.1002/cpph.2. Curr Protoc Pharmacol. 2016. PMID: 27248578 Free PMC article.
-
Cancer-Associated Fibroblasts: Accomplices in the Tumor Immune Evasion.Cancers (Basel). 2020 Oct 14;12(10):2969. doi: 10.3390/cancers12102969. Cancers (Basel). 2020. PMID: 33066357 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

