A review of therapeutic aptamer conjugates with emphasis on new approaches
- PMID: 24276022
- PMCID: PMC3816688
- DOI: 10.3390/ph6030340
A review of therapeutic aptamer conjugates with emphasis on new approaches
Abstract
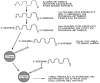
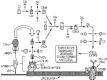
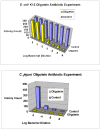
The potential to emulate or enhance antibodies with nucleic acid aptamers while lowering costs has prompted development of new aptamer-protein, siRNA, drug, and nanoparticle conjugates. Specific focal points of this review discuss DNA aptamers covalently bound at their 3' ends to various proteins for enhanced stability and greater pharmacokinetic lifetimes in vivo. The proteins can include Fc tails of IgG for opsonization, and the first component of complement (C1q) to trigger complement-mediated lysis of antibiotic-resistant Gram negative bacteria, cancer cells and possibly some parasites during vulnerable stages. In addition, the 3' protein adduct may be a biotoxin, enzyme, or may simply be human serum albumin (HSA) or a drug known to bind HSA, thereby retarding kidney and other organ clearance and inhibiting serum exonucleases. In this review, the author summarizes existing therapeutic aptamer conjugate categories and describes his patented concept for PCR-based amplification of double-stranded aptamers followed by covalent attachment of proteins or other agents to the chemically vulnerable overhanging 3' adenine added by Taq polymerase. PCR amplification of aptamers could dramatically lower the current $2,000/gram cost of parallel chemical oligonucleotide synthesis, thereby enabling mass production of aptamer-3'-protein or drug conjugates to better compete against expensive humanized monoclonal antibodies.
Figures

Similar articles
-
Oligonucleotide aptamers: new tools for targeted cancer therapy.Mol Ther Nucleic Acids. 2014 Aug 5;3(8):e182. doi: 10.1038/mtna.2014.32. Mol Ther Nucleic Acids. 2014. PMID: 25093706 Free PMC article.
-
Recent advances in understanding oligonucleotide aptamers and their applications as therapeutic agents.3 Biotech. 2020 Dec;10(12):551. doi: 10.1007/s13205-020-02546-1. Epub 2020 Nov 24. 3 Biotech. 2020. PMID: 33269185 Free PMC article. Review.
-
Current Perspectives on Aptamers as Diagnostic Tools and Therapeutic Agents.Pharmaceutics. 2020 Jul 9;12(7):646. doi: 10.3390/pharmaceutics12070646. Pharmaceutics. 2020. PMID: 32659966 Free PMC article. Review.
-
A fluorescent spectroscopy and modelling analysis of anti-heparanase aptamers-serum protein interactions.J Photochem Photobiol B. 2013 Oct 5;127:68-77. doi: 10.1016/j.jphotobiol.2013.06.015. Epub 2013 Aug 3. J Photochem Photobiol B. 2013. PMID: 23968994
-
Using Exonucleases for Aptamer Characterization, Engineering, and Sensing.Acc Chem Res. 2023 Jul 4;56(13):1731-1743. doi: 10.1021/acs.accounts.3c00113. Epub 2023 Jun 14. Acc Chem Res. 2023. PMID: 37314701
Cited by
-
Oligonucleotide aptamer-drug conjugates for targeted therapy of acute myeloid leukemia.Biomaterials. 2015 Oct;67:42-51. doi: 10.1016/j.biomaterials.2015.07.025. Epub 2015 Jul 15. Biomaterials. 2015. PMID: 26204224 Free PMC article.
-
A Highlight of Recent Advances in Aptamer Technology and Its Application.Molecules. 2015 Jun 30;20(7):11959-80. doi: 10.3390/molecules200711959. Molecules. 2015. PMID: 26133761 Free PMC article. Review.
-
Modified internucleoside linkages for nuclease-resistant oligonucleotides.RSC Chem Biol. 2020 Dec 8;2(1):94-150. doi: 10.1039/d0cb00136h. eCollection 2021 Feb 1. RSC Chem Biol. 2020. PMID: 34458777 Free PMC article. Review.
-
Targeting the polyadenylation factor EhCFIm25 with RNA aptamers controls survival in Entamoeba histolytica.Sci Rep. 2018 Apr 9;8(1):5720. doi: 10.1038/s41598-018-23997-w. Sci Rep. 2018. PMID: 29632392 Free PMC article.
-
Preliminary Development of a DNA Aptamer-Magnetic Bead Capture Electrochemiluminescence Sandwich Assay for Brain Natriuretic Peptide.Microchem J. 2014 Jul 1;115:32-38. doi: 10.1016/j.microc.2014.02.003. Microchem J. 2014. PMID: 24764602 Free PMC article.
References
-
- Perez-Ellis C., Goncalves A., Jacquemier J., Marty M., Girre V., Roché H., Brain E., Moatti J.P., Viens P., le Corroller-Soriano A.G. Cost-effectiveness analysis of trastuzumab (Herceptin) in HER2-overexpressed metastatic breast cancer. Am. J. Clin. Oncol. 2009;32:492–498. doi: 10.1097/COC.0b013e3181931277. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

