Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo
- PMID: 24265766
- PMCID: PMC3825691
- DOI: 10.1371/journal.pone.0079325
Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo
Abstract
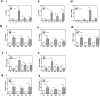
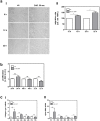
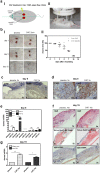
Cold atmospheric plasma (CAP) has the potential to interact with tissue or cells leading to fast, painless and efficient disinfection and furthermore has positive effects on wound healing and tissue regeneration. For clinical implementation it is necessary to examine how CAP improves wound healing and which molecular changes occur after the CAP treatment. In the present study we used the second generation MicroPlaSter ß® in analogy to the current clinical standard (2 min treatment time) in order to determine molecular changes induced by CAP using in vitro cell culture studies with human fibroblasts and an in vivo mouse skin wound healing model. Our in vitro analysis revealed that the CAP treatment induces the expression of important key genes crucial for the wound healing response like IL-6, IL-8, MCP-1, TGF-ß1, TGF-ß2, and promotes the production of collagen type I and alpha-SMA. Scratch wound healing assays showed improved cell migration, whereas cell proliferation analyzed by XTT method, and the apoptotic machinery analyzed by protein array technology, was not altered by CAP in dermal fibroblasts. An in vivo wound healing model confirmed that the CAP treatment affects above mentioned genes involved in wound healing, tissue injury and repair. Additionally, we observed that the CAP treatment improves wound healing in mice, no relevant side effects were detected. We suggest that improved wound healing might be due to the activation of a specified panel of cytokines and growth factors by CAP. In summary, our in vitro human and in vivo animal data suggest that the 2 min treatment with the MicroPlaSter ß® is an effective technique for activating wound healing relevant molecules in dermal fibroblasts leading to improved wound healing, whereas the mechanisms which contribute to these observed effects have to be further investigated.
Conflict of interest statement
Figures
Similar articles
-
Cold atmospheric plasma (CAP) activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode.J Dermatol Sci. 2018 Feb;89(2):181-190. doi: 10.1016/j.jdermsci.2017.11.008. Epub 2017 Nov 26. J Dermatol Sci. 2018. PMID: 29191392
-
Effects of cold atmospheric plasma (CAP) on ß-defensins, inflammatory cytokines, and apoptosis-related molecules in keratinocytes in vitro and in vivo.PLoS One. 2015 Mar 13;10(3):e0120041. doi: 10.1371/journal.pone.0120041. eCollection 2015. PLoS One. 2015. PMID: 25768736 Free PMC article.
-
The HIPPO Transducer YAP and Its Targets CTGF and Cyr61 Drive a Paracrine Signalling in Cold Atmospheric Plasma-Mediated Wound Healing.Oxid Med Cell Longev. 2020 Feb 13;2020:4910280. doi: 10.1155/2020/4910280. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32104533 Free PMC article.
-
[Cold atmospheric pressure plasma for the treatment of acute and chronic wounds].Hautarzt. 2020 Nov;71(11):855-862. doi: 10.1007/s00105-020-04696-y. Hautarzt. 2020. PMID: 32997219 Review. German.
-
Cold atmospheric plasma (CAP): a revolutionary approach in dermatology and skincare.Eur J Med Res. 2024 Oct 5;29(1):487. doi: 10.1186/s40001-024-02088-9. Eur J Med Res. 2024. PMID: 39367460 Free PMC article. Review.
Cited by
-
d-Glucose Oxidation by Cold Atmospheric Plasma-Induced Reactive Species.ACS Omega. 2022 Aug 26;7(36):31983-31998. doi: 10.1021/acsomega.2c02965. eCollection 2022 Sep 13. ACS Omega. 2022. PMID: 36119990 Free PMC article.
-
Improved Wound Healing of Airway Epithelial Cells Is Mediated by Cold Atmospheric Plasma: A Time Course-Related Proteome Analysis.Oxid Med Cell Longev. 2019 May 19;2019:7071536. doi: 10.1155/2019/7071536. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31223425 Free PMC article.
-
Nonthermal Atmospheric Pressure Plasma Treatment of Endosteal Implants for Osseointegration and Antimicrobial Efficacy: A Comprehensive Review.Bioengineering (Basel). 2024 Mar 27;11(4):320. doi: 10.3390/bioengineering11040320. Bioengineering (Basel). 2024. PMID: 38671741 Free PMC article. Review.
-
Comparison of regulatory networks of E74-like factor 1 and cold-shock domain-containing E1 in breast cancer cell lines using ChIP datasets.Exp Ther Med. 2020 Dec;20(6):245. doi: 10.3892/etm.2020.9375. Epub 2020 Oct 22. Exp Ther Med. 2020. PMID: 33178343 Free PMC article.
-
Plasma-liquid interactions in the presence of organic matter-A perspective.J Appl Phys. 2024 Apr 28;135(16):160901. doi: 10.1063/5.0203125. Epub 2024 Apr 26. J Appl Phys. 2024. PMID: 38681528 Free PMC article.
References
-
- Heinlin J, Zimmermann JL, Zeman F, Bunk W, Isbary G, et al... (2013) Randomized placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites. Wound Repair and Regeneration (accepted for publication). - PubMed
-
- Isbary G, Morfill G, Schmidt HU, Georgi M, Ramrath K, et al. (2010) A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br J Dermatol 163: 78–82. - PubMed
-
- Heinlin J, Morfill G, Landthaler M, Stolz W, Isbary G, et al. (2010) Plasma medicine: possible applications in dermatology. J Dtsch Dermatol Ges 8: 968–976. - PubMed
-
- Shimizu T, Steffes B, Pompl R, Jamitzky F, Bunk W, et al... (2008) Characterization of Microwave Plasma Torch for Decontamination. Plasma Process Polym: 5, 577–582.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

