Hippo signaling impedes adult heart regeneration
- PMID: 24255096
- PMCID: PMC3833428
- DOI: 10.1242/dev.102798
Hippo signaling impedes adult heart regeneration
Abstract
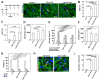
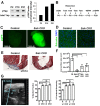
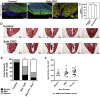
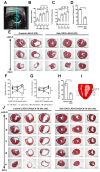
Heart failure due to cardiomyocyte loss after ischemic heart disease is the leading cause of death in the United States in large part because heart muscle regenerates poorly. The endogenous mechanisms preventing mammalian cardiomyocyte regeneration are poorly understood. Hippo signaling, an ancient organ size control pathway, is a kinase cascade that inhibits developing cardiomyocyte proliferation but it has not been studied postnatally or in fully mature adult cardiomyocytes. Here, we investigated Hippo signaling in adult cardiomyocyte renewal and regeneration. We found that unstressed Hippo-deficient adult mouse cardiomyocytes re-enter the cell cycle and undergo cytokinesis. Moreover, Hippo deficiency enhances cardiomyocyte regeneration with functional recovery after adult myocardial infarction as well as after postnatal day eight (P8) cardiac apex resection and P8 myocardial infarction. In damaged hearts, Hippo mutant cardiomyocytes also have elevated proliferation. Our findings reveal that Hippo signaling is an endogenous repressor of adult cardiomyocyte renewal and regeneration. Targeting the Hippo pathway in human disease might be beneficial for the treatment of heart disease.
Keywords: Cell cycle; Mouse; Proliferation; Regeneration.
Figures

Similar articles
-
Actin cytoskeletal remodeling with protrusion formation is essential for heart regeneration in Hippo-deficient mice.Sci Signal. 2015 May 5;8(375):ra41. doi: 10.1126/scisignal.2005781. Sci Signal. 2015. PMID: 25943351 Free PMC article.
-
Upstream regulation of the Hippo-Yap pathway in cardiomyocyte regeneration.Semin Cell Dev Biol. 2020 Apr;100:11-19. doi: 10.1016/j.semcdb.2019.09.004. Epub 2019 Oct 9. Semin Cell Dev Biol. 2020. PMID: 31606277 Free PMC article. Review.
-
A microRNA-Hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice.Sci Transl Med. 2015 Mar 18;7(279):279ra38. doi: 10.1126/scitranslmed.3010841. Sci Transl Med. 2015. PMID: 25787764 Free PMC article.
-
Pitx2 promotes heart repair by activating the antioxidant response after cardiac injury.Nature. 2016 Jun 2;534(7605):119-23. doi: 10.1038/nature17959. Epub 2016 May 25. Nature. 2016. PMID: 27251288 Free PMC article.
-
The cell-autonomous and non-cell-autonomous roles of the Hippo pathway in heart regeneration.J Mol Cell Cardiol. 2022 Jul;168:98-106. doi: 10.1016/j.yjmcc.2022.04.018. Epub 2022 May 5. J Mol Cell Cardiol. 2022. PMID: 35526477 Free PMC article. Review.
Cited by
-
Hippo/Yap Signaling in Cardiac Development and Regeneration.Curr Treat Options Cardiovasc Med. 2016 Jun;18(6):38. doi: 10.1007/s11936-016-0461-y. Curr Treat Options Cardiovasc Med. 2016. PMID: 27040401 Review.
-
A hippo "AKT" regulates cardiomyocyte proliferation.Circ Res. 2015 Jan 2;116(1):3-5. doi: 10.1161/CIRCRESAHA.114.305325. Circ Res. 2015. PMID: 25552685 Free PMC article. No abstract available.
-
Cardiac Regeneration After Myocardial Infarction: an Approachable Goal.Curr Cardiol Rep. 2020 Aug 10;22(10):122. doi: 10.1007/s11886-020-01361-7. Curr Cardiol Rep. 2020. PMID: 32778947 Free PMC article. Review.
-
Actin cytoskeletal remodeling with protrusion formation is essential for heart regeneration in Hippo-deficient mice.Sci Signal. 2015 May 5;8(375):ra41. doi: 10.1126/scisignal.2005781. Sci Signal. 2015. PMID: 25943351 Free PMC article.
-
An injury-responsive mmp14b enhancer is required for heart regeneration.Sci Adv. 2023 Dec;9(48):eadh5313. doi: 10.1126/sciadv.adh5313. Epub 2023 Nov 29. Sci Adv. 2023. PMID: 38019918 Free PMC article.
References
-
- Boon R. A., Iekushi K., Lechner S., Seeger T., Fischer A., Heydt S., Kaluza D., Tréguer K., Carmona G., Bonauer A., et al. (2013). MicroRNA-34a regulates cardiac ageing and function. Nature 495, 107–110 - PubMed
-
- Chugh A. R., Beache G. M., Loughran J. H., Mewton N., Elmore J. B., Kajstura J., Pappas P., Tatooles A., Stoddard M. F., Lima J. A., et al. (2012). Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation 126 Suppl. 1, S54–S64 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

