Targeting chelatable iron as a therapeutic modality in Parkinson's disease
- PMID: 24251381
- PMCID: PMC4060813
- DOI: 10.1089/ars.2013.5593
Targeting chelatable iron as a therapeutic modality in Parkinson's disease
Abstract
Aims: The pathophysiological role of iron in Parkinson's disease (PD) was assessed by a chelation strategy aimed at reducing oxidative damage associated with regional iron deposition without affecting circulating metals. Translational cell and animal models provided concept proofs and a delayed-start (DS) treatment paradigm, the basis for preliminary clinical assessments.
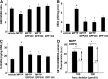
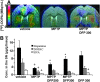
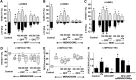
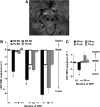
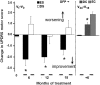
Results: For translational studies, we assessed the effect of oxidative insults in mice systemically prechelated with deferiprone (DFP) by following motor functions, striatal dopamine (HPLC and MRI-PET), and brain iron deposition (relaxation-R2*-MRI) aided by spectroscopic measurements of neuronal labile iron (with fluorescence-sensitive iron sensors) and oxidative damage by markers of protein, lipid, and DNA modification. DFP significantly reduced labile iron and biological damage in oxidation-stressed cells and animals, improving motor functions while raising striatal dopamine. For a pilot, double-blind, placebo-controlled randomized clinical trial, early-stage Parkinson's patients on stabilized dopamine regimens enrolled in a 12-month single-center study with DFP (30 mg/kg/day). Based on a 6-month DS paradigm, early-start patients (n=19) compared to DS patients (n=18) (37/40 completed) responded significantly earlier and sustainably to treatment in both substantia nigra iron deposits (R2* MRI) and Unified Parkinson's Disease Rating Scale motor indicators of disease progression (p<0.03 and p<0.04, respectively). Apart from three rapidly resolved neutropenia cases, safety was maintained throughout the trial.
Innovation: A moderate iron chelation regimen that avoids changes in systemic iron levels may constitute a novel therapeutic modality for PD.
Conclusions: The therapeutic features of a chelation modality established in translational models and in pilot clinical trials warrant comprehensive evaluation of symptomatic and/or disease-modifying potential of chelation in PD.
Trial registration: ClinicalTrials.gov NCT00943748.
Figures


Similar articles
-
Ceruloplasmin activity and iron chelation treatment of patients with Parkinson's disease.BMC Neurol. 2015 May 6;15:74. doi: 10.1186/s12883-015-0331-3. BMC Neurol. 2015. PMID: 25943368 Free PMC article. Clinical Trial.
-
Brain iron chelation by deferiprone in a phase 2 randomised double-blinded placebo controlled clinical trial in Parkinson's disease.Sci Rep. 2017 May 3;7(1):1398. doi: 10.1038/s41598-017-01402-2. Sci Rep. 2017. PMID: 28469157 Free PMC article. Clinical Trial.
-
Trial of Deferiprone in Parkinson's Disease.N Engl J Med. 2022 Dec 1;387(22):2045-2055. doi: 10.1056/NEJMoa2209254. N Engl J Med. 2022. PMID: 36449420 Clinical Trial.
-
Conservative iron chelation for neurodegenerative diseases such as Parkinson's disease and amyotrophic lateral sclerosis.J Neural Transm (Vienna). 2020 Feb;127(2):189-203. doi: 10.1007/s00702-019-02138-1. Epub 2020 Jan 7. J Neural Transm (Vienna). 2020. PMID: 31912279 Review.
-
Efficacy of the iron-chelating agent, deferiprone, in patients with Parkinson's disease: A systematic review and meta-analysis.CNS Neurosci Ther. 2024 Feb;30(2):e14607. doi: 10.1111/cns.14607. CNS Neurosci Ther. 2024. PMID: 38334258 Free PMC article. Review.
Cited by
-
Molecular Mechanisms Underlying Reciprocal Interactions Between Sleep Disorders and Parkinson's Disease.Front Neurosci. 2021 Feb 10;14:592989. doi: 10.3389/fnins.2020.592989. eCollection 2020. Front Neurosci. 2021. PMID: 33642969 Free PMC article. Review.
-
Benefits of Iron Chelators in the Treatment of Parkinson's Disease.Neurochem Res. 2021 May;46(5):1239-1251. doi: 10.1007/s11064-021-03262-9. Epub 2021 Mar 1. Neurochem Res. 2021. PMID: 33646533 Free PMC article.
-
Ceruloplasmin activity and iron chelation treatment of patients with Parkinson's disease.BMC Neurol. 2015 May 6;15:74. doi: 10.1186/s12883-015-0331-3. BMC Neurol. 2015. PMID: 25943368 Free PMC article. Clinical Trial.
-
The value of magnetic resonance imaging as a biomarker for amyotrophic lateral sclerosis: a systematic review.BMC Neurol. 2016 Aug 27;16(1):155. doi: 10.1186/s12883-016-0672-6. BMC Neurol. 2016. PMID: 27567641 Free PMC article. Review.
-
Chelation Combination-A Strategy to Mitigate the Neurotoxicity of Manganese, Iron, and Copper?Biomolecules. 2022 Nov 18;12(11):1713. doi: 10.3390/biom12111713. Biomolecules. 2022. PMID: 36421727 Free PMC article. Review.
References
-
- Boddaert N, Le Quan Sang KH, Rötig A, Leroy-Willig A, Gallet S, Brunelle F, Sidi D, Thalabard JC, Munnich A, and Cabantchik ZI. Selective iron chelation in Friedreich ataxia: biologic and clinical implications. Blood 110: 401–408, 2007 - PubMed
-
- Breuer W, and Cabantchik Z.I. Disorders affecting iron distribution: causes, consequences and possible treatments, Blood Med.com. www.bloodmed.com/800000/mini-reviews1.asp?id=253&p=1&v=1
-
- Breuer W, Shvartsman M, and Cabantchik ZI. Intracellular labile iron. Int J Biochem Cell Biol 40: 350–354, 2008 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

