Phosphorylation of the Rab exchange factor Sec2p directs a switch in regulatory binding partners
- PMID: 24248333
- PMCID: PMC3864272
- DOI: 10.1073/pnas.1320029110
Phosphorylation of the Rab exchange factor Sec2p directs a switch in regulatory binding partners
Abstract
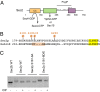
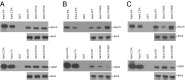
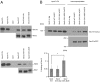
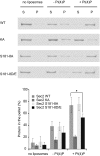
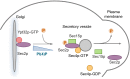
Sec2p is a guanine nucleotide exchange factor that promotes exocytosis by activating the Rab GTPase Sec4p. Sec2p is highly phosphorylated, and we have explored the role of phosphorylation in the regulation of its function. We have identified three phosphosites and demonstrate that phosphorylation regulates the interaction of Sec2p with its binding partners Ypt32p, Sec15p, and phosphatidyl-inositol-4-phosphate. In its nonphosphorylated form, Sec2p binds preferentially to the upstream Rab, Ypt32p-GTP, thus forming a Rab guanine nucleotide exchange factor cascade that leads to the activation of the downstream Rab, Sec4p. The nonphosphorylated form of Sec2p also binds to the Golgi-associated phosphatidyl-inositol-4-phosphate, which works in concert with Ypt32p-GTP to recruit Sec2p to Golgi-derived secretory vesicles. In contrast, the phosphorylated form of Sec2p binds preferentially to Sec15p, a downstream effector of Sec4p and a component of the exocyst tethering complex, thus forming a positive-feedback loop that prepares the secretory vesicle for fusion with the plasma membrane. Our results suggest that the phosphorylation state of Sec2p can direct a switch in its regulatory binding partners that facilitates maturation of the secretory vesicle and helps to promote the directionality of vesicular transport.
Keywords: membrane traffic; phospho-regulation; vesicle maturation; yeast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p.Dev Cell. 2010 May 18;18(5):828-40. doi: 10.1016/j.devcel.2010.03.016. Dev Cell. 2010. PMID: 20493815 Free PMC article.
-
The casein kinases Yck1p and Yck2p act in the secretory pathway, in part, by regulating the Rab exchange factor Sec2p.Mol Biol Cell. 2016 Feb 15;27(4):686-701. doi: 10.1091/mbc.E15-09-0651. Epub 2015 Dec 23. Mol Biol Cell. 2016. PMID: 26700316 Free PMC article.
-
Assaying the interaction of the Rab guanine nucleotide exchange protein Sec2 with the upstream Rab, a downstream effector, and a phosphoinositide.Methods Mol Biol. 2015;1298:85-98. doi: 10.1007/978-1-4939-2569-8_7. Methods Mol Biol. 2015. PMID: 25800834
-
Regulation of membrane traffic by Rab GEF and GAP cascades.Small GTPases. 2016 Oct;7(4):252-256. doi: 10.1080/21541248.2016.1213781. Epub 2016 Jul 18. Small GTPases. 2016. PMID: 27427966 Free PMC article. Review.
-
Rab family of small GTPases: an updated view on their regulation and functions.FEBS J. 2021 Jan;288(1):36-55. doi: 10.1111/febs.15453. Epub 2020 Jul 1. FEBS J. 2021. PMID: 32542850 Free PMC article. Review.
Cited by
-
Profile of Peter Novick.Proc Natl Acad Sci U S A. 2014 Jan 7;111(1):3-4. doi: 10.1073/pnas.1321513110. Epub 2013 Dec 12. Proc Natl Acad Sci U S A. 2014. PMID: 24335703 Free PMC article. No abstract available.
-
Efficient protein production by yeast requires global tuning of metabolism.Nat Commun. 2017 Oct 25;8(1):1131. doi: 10.1038/s41467-017-00999-2. Nat Commun. 2017. PMID: 29070809 Free PMC article.
-
Restoring the Taxol biosynthetic machinery of Aspergillus terreus by Podocarpus gracilior Pilger microbiome, with retrieving the ribosome biogenesis proteins of WD40 superfamily.Sci Rep. 2019 Aug 8;9(1):11534. doi: 10.1038/s41598-019-47816-y. Sci Rep. 2019. PMID: 31395904 Free PMC article.
-
Who's in control? Principles of Rab GTPase activation in endolysosomal membrane trafficking and beyond.J Cell Biol. 2021 Sep 6;220(9):e202105120. doi: 10.1083/jcb.202105120. Epub 2021 Aug 12. J Cell Biol. 2021. PMID: 34383013 Free PMC article. Review.
-
Tracking individual secretory vesicles during exocytosis reveals an ordered and regulated process.J Cell Biol. 2015 Jul 20;210(2):181-9. doi: 10.1083/jcb.201501118. Epub 2015 Jul 13. J Cell Biol. 2015. PMID: 26169352 Free PMC article.
References
-
- Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 2013;93(1):269–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

