Distinct biophysical mechanisms of focal adhesion kinase mechanoactivation by different extracellular matrix proteins
- PMID: 24222685
- PMCID: PMC3845171
- DOI: 10.1073/pnas.1307405110
Distinct biophysical mechanisms of focal adhesion kinase mechanoactivation by different extracellular matrix proteins
Abstract
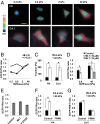
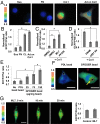
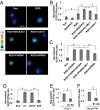
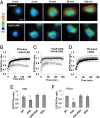
Matrix mechanics controls cell fate by modulating the bonds between integrins and extracellular matrix (ECM) proteins. However, it remains unclear how fibronectin (FN), type 1 collagen, and their receptor integrin subtypes distinctly control force transmission to regulate focal adhesion kinase (FAK) activity, a crucial molecular signal governing cell adhesion/migration. Here we showed, using a genetically encoded FAK biosensor based on fluorescence resonance energy transfer, that FN-mediated FAK activation is dependent on the mechanical tension, which may expose its otherwise hidden FN synergy site to integrin α5. In sharp contrast, the ligation between the constitutively exposed binding motif of type 1 collagen and its receptor integrin α2 was surprisingly tension-independent to induce sufficient FAK activation. Although integrin α subunit determines mechanosensitivity, the ligation between α subunit and the ECM proteins converges at the integrin β1 activation to induce FAK activation. We further discovered that the interaction of the N-terminal protein 4.1/ezrin/redixin/moesin basic patch with phosphatidylinositol 4,5-biphosphate is crucial during cell adhesion to maintain the FAK activation from the inhibitory effect of nearby protein 4.1/ezrin/redixin/moesin acidic sites. Therefore, different ECM proteins either can transmit or can shield from mechanical forces to regulate cellular functions, with the accessibility of ECM binding motifs by their specific integrin α subunits determining the biophysical mechanisms of FAK activation during mechanotransduction.
Keywords: FRET biosensor; intracellular tension; substrate rigidity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Astrocytoma adhesion to extracellular matrix: functional significance of integrin and focal adhesion kinase expression.J Neuropathol Exp Neurol. 1999 Feb;58(2):198-209. doi: 10.1097/00005072-199902000-00009. J Neuropathol Exp Neurol. 1999. PMID: 10029102
-
Simulated physiological stretch increases expression of extracellular matrix proteins in human bladder smooth muscle cells via integrin α4/αv-FAK-ERK1/2 signaling pathway.World J Urol. 2017 Aug;35(8):1247-1254. doi: 10.1007/s00345-016-1993-1. Epub 2016 Dec 24. World J Urol. 2017. PMID: 28013345
-
Activation of focal adhesion kinase enhances the adhesion and invasion of pancreatic cancer cells via extracellular signal-regulated kinase-1/2 signaling pathway activation.Mol Cancer. 2005 Oct 6;4:37. doi: 10.1186/1476-4598-4-37. Mol Cancer. 2005. PMID: 16209712 Free PMC article.
-
Role of focal adhesion kinase in integrin signaling.Int J Biochem Cell Biol. 1997 Aug-Sep;29(8-9):1085-96. doi: 10.1016/s1357-2725(97)00051-4. Int J Biochem Cell Biol. 1997. PMID: 9416004 Review.
-
Endosomes: Emerging Platforms for Integrin-Mediated FAK Signalling.Trends Cell Biol. 2016 Jun;26(6):391-398. doi: 10.1016/j.tcb.2016.02.001. Epub 2016 Mar 2. Trends Cell Biol. 2016. PMID: 26944773 Review.
Cited by
-
Exploring the difference in the mechanics of vascular smooth muscle cells from wild-type and apolipoprotein-E knockout mice.Am J Physiol Cell Physiol. 2022 Nov 1;323(5):C1393-C1401. doi: 10.1152/ajpcell.00046.2022. Epub 2022 Sep 19. Am J Physiol Cell Physiol. 2022. PMID: 36121132 Free PMC article.
-
Integration of mechanical and ECM microenvironment signals in the determination of cancer stem cell states.Curr Stem Cell Rep. 2021 Mar;7:39-47. doi: 10.1007/s40778-020-00182-2. Epub 2020 Nov 23. Curr Stem Cell Rep. 2021. PMID: 33777660 Free PMC article.
-
Viscoelasticity, Like Forces, Plays a Role in Mechanotransduction.Front Cell Dev Biol. 2022 Feb 9;10:789841. doi: 10.3389/fcell.2022.789841. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35223831 Free PMC article. Review.
-
Substrate Stiffness-Dependent Carbon Nanotube-Induced Lung Fibrogenesis.Nano Lett. 2019 Aug 14;19(8):5443-5451. doi: 10.1021/acs.nanolett.9b01943. Epub 2019 Aug 5. Nano Lett. 2019. PMID: 31369708 Free PMC article.
-
Epidermal ROCK2 induces AKT1/GSK3β/β-catenin, NFκB and dermal tenascin C; but enhanced differentiation and p53/p21 inhibit papilloma.Carcinogenesis. 2020 Oct 15;41(10):1409-1420. doi: 10.1093/carcin/bgz205. Carcinogenesis. 2020. PMID: 31907522 Free PMC article.
References
-
- Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10(1):75–82. - PubMed
-
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. - PubMed
-
- Trappmann B, et al. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11(7):642–649. - PubMed
-
- Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

