Negative impact of β-arrestin-1 on post-myocardial infarction heart failure via cardiac and adrenal-dependent neurohormonal mechanisms
- PMID: 24218435
- PMCID: PMC3889868
- DOI: 10.1161/HYPERTENSIONAHA.113.02043
Negative impact of β-arrestin-1 on post-myocardial infarction heart failure via cardiac and adrenal-dependent neurohormonal mechanisms
Abstract
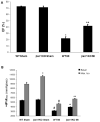
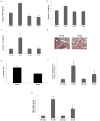
β-Arrestin (βarr)-1 and β-arrestin-2 (βarrs) are universal G-protein-coupled receptor adapter proteins that negatively regulate cardiac β-adrenergic receptor (βAR) function via βAR desensitization and downregulation. In addition, they mediate G-protein-independent βAR signaling, which might be beneficial, for example, antiapoptotic, for the heart. However, the specific role(s) of each βarr isoform in cardiac βAR dysfunction, the molecular hallmark of chronic heart failure (HF), remains unknown. Furthermore, adrenal βarr1 exacerbates HF by chronically enhancing adrenal production and hence circulating levels of aldosterone and catecholamines. Herein, we sought to delineate specific roles of βarr1 in post-myocardial infarction (MI) HF by testing the effects of βarr1 genetic deletion on normal and post-MI cardiac function and morphology. We studied βarr1 knockout (βarr1KO) mice alongside wild-type controls under normal conditions and after surgical MI. Normal (sham-operated) βarr1KO mice display enhanced βAR-dependent contractility and post-MI βarr1KO mice enhanced overall cardiac function (and βAR-dependent contractility) compared with wild type. Post-MI βarr1KO mice also show increased survival and decreased cardiac infarct size, apoptosis, and adverse remodeling, as well as circulating catecholamines and aldosterone, compared with post-MI wild type. The underlying mechanisms, on one hand, improved cardiac βAR signaling and function, as evidenced by increased βAR density and procontractile signaling, via reduced cardiac βAR desensitization because of cardiac βarr1 absence, and, on the other hand, decreased production leading to lower circulating levels of catecholamines and aldosterone because of adrenal βarr1 absence. Thus, βarr1, via both cardiac and adrenal effects, is detrimental for cardiac structure and function and significantly exacerbates post-MI HF.
Keywords: aldosterone; catecholamines; knockout mice; β-arrestin-1.
Conflict of interest statement
The authors declare no relationships with industry or any other conflict of interest.
Figures
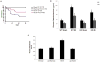


Comment in
-
Adrenal signaling in heart failure: something more than a distant ship's smoke on the horizon.Hypertension. 2014 Feb;63(2):215-6. doi: 10.1161/HYPERTENSIONAHA.113.02382. Epub 2013 Nov 11. Hypertension. 2014. PMID: 24218430 No abstract available.
Similar articles
-
Adrenal beta-arrestin 1 inhibition in vivo attenuates post-myocardial infarction progression to heart failure and adverse remodeling via reduction of circulating aldosterone levels.J Am Coll Cardiol. 2011 Jan 18;57(3):356-65. doi: 10.1016/j.jacc.2010.08.635. J Am Coll Cardiol. 2011. PMID: 21232674 Free PMC article.
-
β-Arrestin2 Improves Post-Myocardial Infarction Heart Failure via Sarco(endo)plasmic Reticulum Ca2+-ATPase-Dependent Positive Inotropy in Cardiomyocytes.Hypertension. 2017 Nov;70(5):972-981. doi: 10.1161/HYPERTENSIONAHA.117.09817. Epub 2017 Sep 5. Hypertension. 2017. PMID: 28874462
-
An adrenal beta-arrestin 1-mediated signaling pathway underlies angiotensin II-induced aldosterone production in vitro and in vivo.Proc Natl Acad Sci U S A. 2009 Apr 7;106(14):5825-30. doi: 10.1073/pnas.0811706106. Epub 2009 Mar 16. Proc Natl Acad Sci U S A. 2009. PMID: 19289825 Free PMC article.
-
Therapeutic Targets for Treatment of Heart Failure: Focus on GRKs and β-Arrestins Affecting βAR Signaling.Front Pharmacol. 2018 Nov 27;9:1336. doi: 10.3389/fphar.2018.01336. eCollection 2018. Front Pharmacol. 2018. PMID: 30538631 Free PMC article. Review.
-
βArrestins in cardiac G protein-coupled receptor signaling and function: partners in crime or "good cop, bad cop"?Int J Mol Sci. 2013 Dec 18;14(12):24726-41. doi: 10.3390/ijms141224726. Int J Mol Sci. 2013. PMID: 24351844 Free PMC article. Review.
Cited by
-
Adrenal βarrestin1 targeting for tobacco-associated cardiac dysfunction treatment: Aldosterone production as the mechanistic link.Pharmacol Res Perspect. 2019 Jun 18;7(4):e00497. doi: 10.1002/prp2.497. eCollection 2019 Aug. Pharmacol Res Perspect. 2019. PMID: 31236278 Free PMC article. Review.
-
Angiotensin receptor blocker drugs and inhibition of adrenal beta-arrestin-1-dependent aldosterone production: Implications for heart failure therapy.World J Cardiol. 2017 Mar 26;9(3):200-206. doi: 10.4330/wjc.v9.i3.200. World J Cardiol. 2017. PMID: 28400916 Free PMC article.
-
Insights Into the Role of Angiotensin-II AT1 Receptor-Dependent β-Arrestin Signaling in Cardiovascular Disease.Hypertension. 2024 Jan;81(1):6-16. doi: 10.1161/HYPERTENSIONAHA.123.19419. Epub 2023 Jul 14. Hypertension. 2024. PMID: 37449411 Free PMC article. Review.
-
ARRB1 ameliorates liver ischaemia/reperfusion injury via antagonizing TRAF6-mediated Lysine 6-linked polyubiquitination of ASK1 in hepatocytes.J Cell Mol Med. 2020 Jul;24(14):7814-7828. doi: 10.1111/jcmm.15412. Epub 2020 May 23. J Cell Mol Med. 2020. PMID: 32445435 Free PMC article.
-
Regulation of CaMKII signaling in cardiovascular disease.Front Pharmacol. 2015 Aug 25;6:178. doi: 10.3389/fphar.2015.00178. eCollection 2015. Front Pharmacol. 2015. PMID: 26379551 Free PMC article. Review.
References
-
- Tamargo J, López-Sendón J. Novel therapeutic targets for the treatment of heart failure. Nat Rev Drug Disc. 2011;10:536–555. - PubMed
-
- Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. - PubMed
-
- Brodde OE. Beta-adrenoceptors in cardiac disease. Pharmacol Ther. 1993;60:405–430. - PubMed
-
- Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. - PubMed
-
- Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and β-adrenegic receptor density in failing human hearts. N Engl J Med. 1982;307:205–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

