Molecular determinants of orexin receptor-arrestin-ubiquitin complex formation
- PMID: 24206104
- PMCID: PMC3904257
- DOI: 10.1111/bph.12481
Molecular determinants of orexin receptor-arrestin-ubiquitin complex formation
Abstract
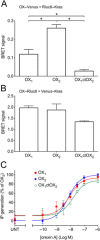
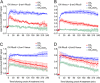
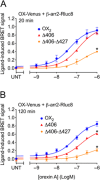
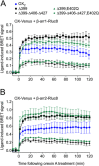
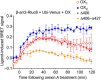
Background and purpose: The orexin system regulates a multitude of key physiological processes, particularly involving maintenance of metabolic homeostasis. Consequently, there is considerable potential for pharmaceutical development for the treatment of disorders from narcolepsy to metabolic syndrome. It acts through the hormonal activity of two endogenous peptides, orexin A binding to orexin receptors 1 and 2 (OX₁ and OX₂) with similar affinity, and orexin B binding to OX₂ with higher affinity than OX₁ receptors. We have previously revealed data differentiating orexin receptor subtypes with respect to their relative stability in forming orexin receptor-arrestin-ubiquitin complexes measured by BRET. Recycling and cellular signalling distinctions were also observed. Here, we have investigated, using BRET, the molecular determinants involved in providing OX₂ receptors with greater β-arrestin-ubiquitin complex stability.
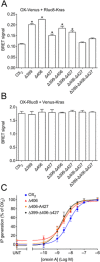
Experimental approach: The contribution of the C-terminal tail of the OX receptors was investigated by bulk substitution and site-specific mutagenesis using BRET and inositol phosphate assays.
Key results: Replacement of the OX₁ receptor C-terminus with that of the OX₂ receptor did not result in the expected gain of function, indicating a role for intracellular domain configuration in addition to primary structure. Furthermore, two out of the three putative serine/threonine clusters in the C-terminus were found to be involved in OX₂ receptor-β-arrestin-ubiquitin complex formation.
Conclusions and implications: This study provides fundamental insights into the molecular elements that influence receptor-arrestin-ubiquitin complex formation. Understanding how and why the orexin receptors can be functionally differentiated brings us closer to exploiting these receptors as drug targets.
Keywords: BRET; GPCR; arrestin; hypocretin; orexin; ubiquitin.
© 2013 The Authors. British Journal of Pharmacology published by John Wiley &. Sons Ltd on behalf of The British Pharmacological Society.
Figures



Similar articles
-
Temporal profiling of orexin receptor-arrestin-ubiquitin complexes reveals differences between receptor subtypes.J Biol Chem. 2011 May 13;286(19):16726-33. doi: 10.1074/jbc.M111.223537. Epub 2011 Mar 4. J Biol Chem. 2011. PMID: 21378163 Free PMC article.
-
Biochemical and behavioural characterization of EMPA, a novel high-affinity, selective antagonist for the OX(2) receptor.Br J Pharmacol. 2009 Apr;156(8):1326-41. doi: 10.1111/j.1476-5381.2009.00127.x. Br J Pharmacol. 2009. PMID: 19751316 Free PMC article.
-
Toward an understanding of agonist binding to human Orexin-1 and Orexin-2 receptors with G-protein-coupled receptor modeling and site-directed mutagenesis.Biochemistry. 2013 Nov 19;52(46):8246-60. doi: 10.1021/bi401119m. Epub 2013 Nov 8. Biochemistry. 2013. PMID: 24144388 Free PMC article.
-
The physiology and pharmacology of the orexins.Pharmacol Ther. 2002 Apr-May;94(1-2):51-61. doi: 10.1016/s0163-7258(02)00171-7. Pharmacol Ther. 2002. PMID: 12191593 Review.
-
Dysregulation of the orexin/hypocretin system is not limited to narcolepsy but has far-reaching implications for neurological disorders.Eur J Neurosci. 2021 Feb;53(4):1136-1154. doi: 10.1111/ejn.15077. Epub 2020 Dec 24. Eur J Neurosci. 2021. PMID: 33290595 Review.
Cited by
-
Mutations of Vasopressin Receptor 2 Including Novel L312S Have Differential Effects on Trafficking.Mol Endocrinol. 2016 Aug;30(8):889-904. doi: 10.1210/me.2016-1002. Epub 2016 Jun 29. Mol Endocrinol. 2016. PMID: 27355191 Free PMC article.
-
Fluorescence- and bioluminescence-based approaches to study GPCR ligand binding.Br J Pharmacol. 2016 Oct;173(20):3028-37. doi: 10.1111/bph.13316. Epub 2015 Nov 5. Br J Pharmacol. 2016. PMID: 26317175 Free PMC article. Review.
-
α-Synuclein Induced the Occurrence of RBD via Interaction with OX1R and Modulated Its Degradation.Neuromolecular Med. 2023 Jun;25(2):286-300. doi: 10.1007/s12017-023-08735-4. Epub 2023 Jan 23. Neuromolecular Med. 2023. PMID: 36689149
-
Orexin Receptor Multimerization versus Functional Interactions: Neuropharmacological Implications for Opioid and Cannabinoid Signalling and Pharmacogenetics.Pharmaceuticals (Basel). 2017 Oct 8;10(4):79. doi: 10.3390/ph10040079. Pharmaceuticals (Basel). 2017. PMID: 28991183 Free PMC article.
-
Orexin/hypocretin based pharmacotherapies for the treatment of addiction: DORA or SORA?CNS Drugs. 2014 Aug;28(8):713-30. doi: 10.1007/s40263-014-0179-x. CNS Drugs. 2014. PMID: 24942635 Review.
References
-
- Anborgh PH, Seachrist JL, Dale LB, Ferguson SS. Receptor/beta-arrestin complex formation and the differential trafficking and resensitization of beta2-adrenergic and angiotensin II type 1A receptors. Mol Endocrinol. 2000;14:2040–2053. - PubMed
-
- DeWire S, Ahn S, Lefkowitz R, Shenoy S. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. - PubMed
-
- Evans NA, Groarke DA, Warrack J, Greenwood CJ, Dodgson K, Milligan G, et al. Visualizing differences in ligand-induced beta-arrestin-GFP interactions and trafficking between three recently characterized G protein-coupled receptors. J Neurochem. 2001;77:476–485. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

