Nuclear Fragile X Mental Retardation Protein is localized to Cajal bodies
- PMID: 24204304
- PMCID: PMC3814324
- DOI: 10.1371/journal.pgen.1003890
Nuclear Fragile X Mental Retardation Protein is localized to Cajal bodies
Abstract
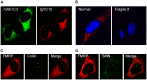
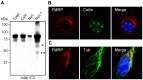
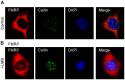
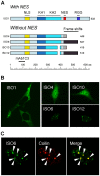
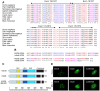
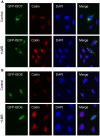
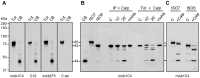
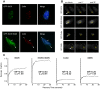
Fragile X syndrome is caused by loss of function of a single gene encoding the Fragile X Mental Retardation Protein (FMRP). This RNA-binding protein, widely expressed in mammalian tissues, is particularly abundant in neurons and is a component of messenger ribonucleoprotein (mRNP) complexes present within the translational apparatus. The absence of FMRP in neurons is believed to cause translation dysregulation and defects in mRNA transport essential for local protein synthesis and for synaptic development and maturation. A prevalent model posits that FMRP is a nucleocytoplasmic shuttling protein that transports its mRNA targets from the nucleus to the translation machinery. However, it is not known which of the multiple FMRP isoforms, resulting from the numerous alternatively spliced FMR1 transcripts variants, would be involved in such a process. Using a new generation of anti-FMRP antibodies and recombinant expression, we show here that the most commonly expressed human FMRP isoforms (ISO1 and 7) do not localize to the nucleus. Instead, specific FMRP isoforms 6 and 12 (ISO6 and 12), containing a novel C-terminal domain, were the only isoforms that localized to the nuclei in cultured human cells. These isoforms localized to specific p80-coilin and SMN positive structures that were identified as Cajal bodies. The Cajal body localization signal was confined to a 17 amino acid stretch in the C-terminus of human ISO6 and is lacking in a mouse Iso6 variant. As FMRP is an RNA-binding protein, its presence in Cajal bodies suggests additional functions in nuclear post-transcriptional RNA metabolism. Supporting this hypothesis, a missense mutation (I304N), known to alter the KH2-mediated RNA binding properties of FMRP, abolishes the localization of human FMRP ISO6 to Cajal bodies. These findings open unexplored avenues in search for new insights into the pathophysiology of Fragile X Syndrome.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Expression of fragile X mental retardation-1 gene with nuclear export signal mutation changes the expression profiling of mouse cerebella immortal neuronal cell.Proteomics. 2005 Oct;5(15):3979-90. doi: 10.1002/pmic.200401252. Proteomics. 2005. PMID: 16130171
-
Absence of the Fragile X Mental Retardation Protein results in defects of RNA editing of neuronal mRNAs in mouse.RNA Biol. 2017 Nov 2;14(11):1580-1591. doi: 10.1080/15476286.2017.1338232. Epub 2017 Sep 5. RNA Biol. 2017. PMID: 28640668 Free PMC article.
-
Splicing of exon 9a in FMR1 transcripts results in a truncated FMRP with altered subcellular distribution.Gene. 2020 Mar 20;731:144359. doi: 10.1016/j.gene.2020.144359. Epub 2020 Jan 11. Gene. 2020. PMID: 31935509
-
FMRP ribonucleoprotein complexes and RNA homeostasis.Adv Genet. 2020;105:95-136. doi: 10.1016/bs.adgen.2020.01.001. Epub 2020 Feb 6. Adv Genet. 2020. PMID: 32560791 Review.
-
New insights into fragile X syndrome: from molecules to neurobehaviors.Trends Biochem Sci. 2003 Mar;28(3):152-8. doi: 10.1016/S0968-0004(03)00033-1. Trends Biochem Sci. 2003. PMID: 12633995 Review.
Cited by
-
An "Omic" Overview of Fragile X Syndrome.Biology (Basel). 2021 May 13;10(5):433. doi: 10.3390/biology10050433. Biology (Basel). 2021. PMID: 34068266 Free PMC article. Review.
-
The translational regulator FMRP controls lipid and glucose metabolism in mice and humans.Mol Metab. 2019 Mar;21:22-35. doi: 10.1016/j.molmet.2019.01.002. Epub 2019 Jan 14. Mol Metab. 2019. PMID: 30686771 Free PMC article.
-
Single cell transcriptomics reveals dysregulated cellular and molecular networks in a fragile X syndrome model.PLoS Genet. 2022 Jun 8;18(6):e1010221. doi: 10.1371/journal.pgen.1010221. eCollection 2022 Jun. PLoS Genet. 2022. PMID: 35675353 Free PMC article.
-
FMRP deficiency leads to multifactorial dysregulation of splicing and mislocalization of MBNL1 to the cytoplasm.PLoS Biol. 2023 Dec 4;21(12):e3002417. doi: 10.1371/journal.pbio.3002417. eCollection 2023 Dec. PLoS Biol. 2023. PMID: 38048343 Free PMC article.
-
Reduced phenotypic severity following adeno-associated virus-mediated Fmr1 gene delivery in fragile X mice.Neuropsychopharmacology. 2014 Dec;39(13):3100-11. doi: 10.1038/npp.2014.167. Epub 2014 Jul 7. Neuropsychopharmacology. 2014. PMID: 24998620 Free PMC article.
References
-
- O'Donnell WT, Warren ST (2002) A decade of molecular studies of fragile X syndrome. Annu Rev Neurosci 25: 315–338. - PubMed
-
- Bardoni B, Davidovic L, Bensaid M, Khandjian EW (2006) The fragile X syndrome: exploring its molecular basis and seeking a treatment. Expert Rev Mol Med 8: 1–16. - PubMed
-
- Devys D, Lutz Y, Rouyer N, Bellocq J-P, Mandel J-L (1993) The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genet 4: 335–340. - PubMed
-
- Siomi H, Siomi MC, Nussbaum RL, Dreyfuss G (1993) The protein product of the fragile X gene, FMR1, has characteristics of an RNA-binding protein. Cell 74: 291–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

