Mycobacterium tuberculosis type VII secreted effector EsxH targets host ESCRT to impair trafficking
- PMID: 24204276
- PMCID: PMC3814348
- DOI: 10.1371/journal.ppat.1003734
Mycobacterium tuberculosis type VII secreted effector EsxH targets host ESCRT to impair trafficking
Abstract
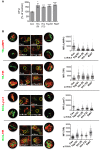
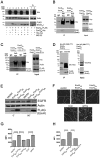
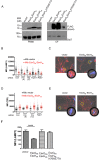
Mycobacterium tuberculosis (Mtb) disrupts anti-microbial pathways of macrophages, cells that normally kill bacteria. Over 40 years ago, D'Arcy Hart showed that Mtb avoids delivery to lysosomes, but the molecular mechanisms that allow Mtb to elude lysosomal degradation are poorly understood. Specialized secretion systems are often used by bacterial pathogens to translocate effectors that target the host, and Mtb encodes type VII secretion systems (TSSSs) that enable mycobacteria to secrete proteins across their complex cell envelope; however, their cellular targets are unknown. Here, we describe a systematic strategy to identify bacterial virulence factors by looking for interactions between the Mtb secretome and host proteins using a high throughput, high stringency, yeast two-hybrid (Y2H) platform. Using this approach we identified an interaction between EsxH, which is secreted by the Esx-3 TSSS, and human hepatocyte growth factor-regulated tyrosine kinase substrate (Hgs/Hrs), a component of the endosomal sorting complex required for transport (ESCRT). ESCRT has a well-described role in directing proteins destined for lysosomal degradation into intraluminal vesicles (ILVs) of multivesicular bodies (MVBs), ensuring degradation of the sorted cargo upon MVB-lysosome fusion. Here, we show that ESCRT is required to deliver Mtb to the lysosome and to restrict intracellular bacterial growth. Further, EsxH, in complex with EsxG, disrupts ESCRT function and impairs phagosome maturation. Thus, we demonstrate a role for a TSSS and the host ESCRT machinery in one of the central features of tuberculosis pathogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Mycobacterium tuberculosis Type VII Secretion System Effectors Differentially Impact the ESCRT Endomembrane Damage Response.mBio. 2018 Nov 27;9(6):e01765-18. doi: 10.1128/mBio.01765-18. mBio. 2018. PMID: 30482832 Free PMC article.
-
Mycobacterium tuberculosis EsxH inhibits ESCRT-dependent CD4+ T-cell activation.Nat Microbiol. 2016 Dec 5;2:16232. doi: 10.1038/nmicrobiol.2016.232. Nat Microbiol. 2016. PMID: 27918526 Free PMC article.
-
The SecA2 pathway of Mycobacterium tuberculosis exports effectors that work in concert to arrest phagosome and autophagosome maturation.PLoS Pathog. 2018 Apr 30;14(4):e1007011. doi: 10.1371/journal.ppat.1007011. eCollection 2018 Apr. PLoS Pathog. 2018. PMID: 29709019 Free PMC article.
-
ESCRT and Membrane Protein Ubiquitination.Prog Mol Subcell Biol. 2018;57:107-135. doi: 10.1007/978-3-319-96704-2_4. Prog Mol Subcell Biol. 2018. PMID: 30097773 Review.
-
Mechanisms of immune evasion by Mycobacterium tuberculosis: the impact of T7SS and cell wall lipids on host defenses.Crit Rev Biochem Mol Biol. 2024 Oct;59(5):310-336. doi: 10.1080/10409238.2024.2411264. Epub 2024 Oct 8. Crit Rev Biochem Mol Biol. 2024. PMID: 39378051 Review.
Cited by
-
Extracellular vesicles: masters of intercellular communication and potential clinical interventions.J Clin Invest. 2016 Apr 1;126(4):1139-43. doi: 10.1172/JCI87316. Epub 2016 Apr 1. J Clin Invest. 2016. PMID: 27035805 Free PMC article. Review.
-
Propulsive cell entry diverts pathogens from immune degradation by remodeling the phagocytic synapse.Proc Natl Acad Sci U S A. 2023 Dec 5;120(49):e2306788120. doi: 10.1073/pnas.2306788120. Epub 2023 Nov 30. Proc Natl Acad Sci U S A. 2023. PMID: 38032935 Free PMC article.
-
Mycobacterium tuberculosis Type VII Secretion System Effectors Differentially Impact the ESCRT Endomembrane Damage Response.mBio. 2018 Nov 27;9(6):e01765-18. doi: 10.1128/mBio.01765-18. mBio. 2018. PMID: 30482832 Free PMC article.
-
Transcriptome Changes of Mycobacterium marinum in the Process of Resuscitation From Hypoxia-Induced Dormancy.Front Genet. 2020 Feb 7;10:1359. doi: 10.3389/fgene.2019.01359. eCollection 2019. Front Genet. 2020. PMID: 32117415 Free PMC article.
-
Mycobacterium tuberculosis strain with deletions in menT3 and menT4 is attenuated and confers protection in mice and guinea pigs.Nat Commun. 2024 Jun 27;15(1):5467. doi: 10.1038/s41467-024-49246-5. Nat Commun. 2024. PMID: 38937463 Free PMC article.
References
-
- Russell DG (2001) Mycobacterium tuberculosis: here today, and here tomorrow. Nat Rev Mol Cell Biol 2: 569–577. - PubMed
-
- Flannagan RS, Cosío G, Grinstein S (2009) Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol 7: 355–366. - PubMed
-
- Philips JA, Ernst JD (2012) Tuberculosis pathogenesis and immunity. Annu Rev Pathol 7: 353–384. - PubMed
-
- van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, et al. (2007) M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 129: 1287–1298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

