Modeling Mycobacterium tuberculosis early granuloma formation in experimental human lung tissue
- PMID: 24203885
- PMCID: PMC3917249
- DOI: 10.1242/dmm.013854
Modeling Mycobacterium tuberculosis early granuloma formation in experimental human lung tissue
Abstract
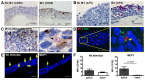
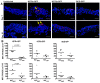
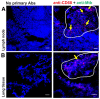
The widely used animal models for tuberculosis (TB) display fundamental differences from human TB. Therefore, a validated model that recapitulates human lung TB is attractive for TB research. Here, we describe a unique method for establishment of TB infection in an experimental human lung tissue model. The model is based on cell lines derived from human lungs and primary macrophages from peripheral blood, and displays characteristics of human lung tissue, including evenly integrated macrophages throughout the epithelium, production of extracellular matrix, stratified epithelia and mucus secretion. Establishment of experimental infection in the model tissue with Mycobacterium tuberculosis, the bacterium that causes TB, resulted in clustering of macrophages at the site of infection, reminiscent of early TB granuloma formation. We quantitated the extent of granuloma formation induced by different strains of mycobacteria and validated our model against findings in other TB models. We found that early granuloma formation is dependent on ESAT-6, which is secreted via the type VII secretion machinery of virulent mycobacteria. Our model, which can facilitate the discovery of the interactions between mycobacteria and host cells in a physiological environment, is the first lung tissue model described for TB.
Keywords: ESX-1; Granuloma; In vitro model; Lung tissue; M. tuberculosis; Tuberculosis.
Figures

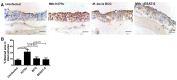
Similar articles
-
Establishment of a Patient-Derived, Magnetic Levitation-Based, Three-Dimensional Spheroid Granuloma Model for Human Tuberculosis.mSphere. 2021 Aug 25;6(4):e0055221. doi: 10.1128/mSphere.00552-21. Epub 2021 Jul 21. mSphere. 2021. PMID: 34287004 Free PMC article.
-
Understanding the pathophysiology of the human TB lung granuloma using in vitro granuloma models.Future Microbiol. 2016 Aug;11:1073-89. doi: 10.2217/fmb-2016-0005. Epub 2016 Aug 9. Future Microbiol. 2016. PMID: 27501829 Review.
-
A 3D Human Lung Tissue Model for Functional Studies on Mycobacterium tuberculosis Infection.J Vis Exp. 2015 Oct 5;(104):53084. doi: 10.3791/53084. J Vis Exp. 2015. PMID: 26485646 Free PMC article.
-
Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium.Science. 2010 Jan 22;327(5964):466-9. doi: 10.1126/science.1179663. Epub 2009 Dec 10. Science. 2010. PMID: 20007864 Free PMC article.
-
Who puts the tubercle in tuberculosis?Nat Rev Microbiol. 2007 Jan;5(1):39-47. doi: 10.1038/nrmicro1538. Epub 2006 Dec 11. Nat Rev Microbiol. 2007. PMID: 17160001 Review.
Cited by
-
Inhibition of Tissue Matrix Metalloproteinases Interferes with Mycobacterium tuberculosis-Induced Granuloma Formation and Reduces Bacterial Load in a Human Lung Tissue Model.Front Microbiol. 2017 Dec 5;8:2370. doi: 10.3389/fmicb.2017.02370. eCollection 2017. Front Microbiol. 2017. PMID: 29259583 Free PMC article.
-
Infect and Inject: How Mycobacterium tuberculosis Exploits Its Major Virulence-Associated Type VII Secretion System, ESX-1.Microbiol Spectr. 2019 May;7(3):10.1128/microbiolspec.bai-0024-2019. doi: 10.1128/microbiolspec.BAI-0024-2019. Microbiol Spectr. 2019. PMID: 31172908 Free PMC article. Review.
-
Macrophage immunoregulatory pathways in tuberculosis.Semin Immunol. 2014 Dec;26(6):471-85. doi: 10.1016/j.smim.2014.09.010. Epub 2014 Oct 30. Semin Immunol. 2014. PMID: 25453226 Free PMC article. Review.
-
Establishment of a Patient-Derived, Magnetic Levitation-Based, Three-Dimensional Spheroid Granuloma Model for Human Tuberculosis.mSphere. 2021 Aug 25;6(4):e0055221. doi: 10.1128/mSphere.00552-21. Epub 2021 Jul 21. mSphere. 2021. PMID: 34287004 Free PMC article.
-
Antigen identification strategies and preclinical evaluation models for advancing tuberculosis vaccine development.NPJ Vaccines. 2024 Mar 9;9(1):57. doi: 10.1038/s41541-024-00834-y. NPJ Vaccines. 2024. PMID: 38461350 Free PMC article. Review.
References
-
- Abdallah A. M., Bestebroer J., Savage N. D., de Punder K., van Zon M., Wilson L., Korbee C. J., van der Sar A. M., Ottenhoff T. H., van der Wel N. N., et al. (2011). Mycobacterial secretion systems ESX-1 and ESX-5 play distinct roles in host cell death and inflammasome activation. J. Immunol. 187, 4744–4753 - PubMed
-
- Brighenti S., Andersson J. (2012). Local immune responses in human tuberculosis: learning from the site of infection. J. Infect. Dis. 205 Suppl., S316–S324 - PubMed
-
- Cozens A. L., Yezzi M. J., Kunzelmann K., Ohrui T., Chin L., Eng K., Finkbeiner W. E., Widdicombe J. H., Gruenert D. C. (1994). CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 10, 38–47 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

