PML isoforms in response to arsenic: high-resolution analysis of PML body structure and degradation
- PMID: 24190887
- PMCID: PMC3889398
- DOI: 10.1242/jcs.132290
PML isoforms in response to arsenic: high-resolution analysis of PML body structure and degradation
Abstract
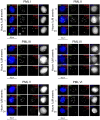
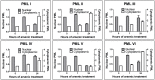
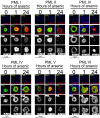
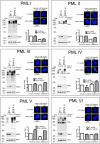
Arsenic is a clinically effective treatment for acute promyelocytic leukaemia (APL) in which the promyelocytic leukaemia (PML) protein is fused to retinoic receptor alpha (RARα). PML-RARα is degraded by the proteasome by a SUMO-dependent, ubiquitin-mediated pathway in response to arsenic treatment, curing the disease. Six major PML isoforms are expressed as a result of alternative splicing, each of which encodes a unique C-terminal region. Using a system in which only a single EYFP-linked PML isoform is expressed, we demonstrate that PMLI, PMLII and PMLVI accumulate in the cytoplasm following arsenic treatment, whereas PMLIII, PMLIV and PMLV do not. 3D structured illumination was used to obtain super-resolution images of PML bodies, revealing spherical shells of PML along with associated SUMO. Arsenic treatment results in dramatic isoform-specific changes to PML body ultrastructure. After extended arsenic treatment most PML isoforms are degraded, leaving SUMO at the core of the nuclear bodies. A high-content imaging assay identifies PMLV as the isoform most readily degraded following arsenic treatment, and PMLIV as relatively resistant to degradation. Immunoprecipitation analysis demonstrates that all PML isoforms are modified by SUMO and ubiquitin after arsenic treatment, and by using siRNA, we demonstrate that arsenic-induced degradation of all PML isoforms is dependent on the ubiquitin E3 ligase RNF4. Intriguingly, depletion of RNF4 results in marked accumulation of PMLV, suggesting that this isoform is an optimal substrate for RNF4. Thus the variable C-terminal domain influences the rate and location of degradation of PML isoforms following arsenic treatment.
Keywords: Arsenic; PML; RNF4; SUMO.
Figures


Similar articles
-
Arsenic-induced SUMO-dependent recruitment of RNF4 into PML nuclear bodies.Mol Biol Cell. 2010 Dec;21(23):4227-39. doi: 10.1091/mbc.E10-05-0449. Epub 2010 Oct 13. Mol Biol Cell. 2010. PMID: 20943951 Free PMC article.
-
Requirement of PML SUMO interacting motif for RNF4- or arsenic trioxide-induced degradation of nuclear PML isoforms.PLoS One. 2012;7(9):e44949. doi: 10.1371/journal.pone.0044949. Epub 2012 Sep 18. PLoS One. 2012. PMID: 23028697 Free PMC article.
-
RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation.Nat Cell Biol. 2008 May;10(5):538-46. doi: 10.1038/ncb1716. Epub 2008 Apr 13. Nat Cell Biol. 2008. PMID: 18408734
-
Role and fate of PML nuclear bodies in response to interferon and viral infections.Oncogene. 2001 Oct 29;20(49):7274-86. doi: 10.1038/sj.onc.1204854. Oncogene. 2001. PMID: 11704856 Review.
-
Pathways of retinoic acid- or arsenic trioxide-induced PML/RARalpha catabolism, role of oncogene degradation in disease remission.Oncogene. 2001 Oct 29;20(49):7257-65. doi: 10.1038/sj.onc.1204852. Oncogene. 2001. PMID: 11704854 Review.
Cited by
-
Superresolution light microscopy of the Drosophila histone locus body reveals a core-shell organization associated with expression of replication-dependent histone genes.Mol Biol Cell. 2021 Apr 19;32(9):942-955. doi: 10.1091/mbc.E20-10-0645. Epub 2021 Mar 31. Mol Biol Cell. 2021. PMID: 33788585 Free PMC article.
-
Generation of specific inhibitors of SUMO-1- and SUMO-2/3-mediated protein-protein interactions using Affimer (Adhiron) technology.Sci Signal. 2017 Nov 14;10(505):eaaj2005. doi: 10.1126/scisignal.aaj2005. Sci Signal. 2017. PMID: 29138295 Free PMC article.
-
On the Prevalence and Roles of Proteins Undergoing Liquid-Liquid Phase Separation in the Biogenesis of PML-Bodies.Biomolecules. 2023 Dec 18;13(12):1805. doi: 10.3390/biom13121805. Biomolecules. 2023. PMID: 38136675 Free PMC article.
-
Reorganization of Cell Compartmentalization Induced by Stress.Biomolecules. 2022 Oct 8;12(10):1441. doi: 10.3390/biom12101441. Biomolecules. 2022. PMID: 36291650 Free PMC article. Review.
-
SUMO deconjugation is required for arsenic-triggered ubiquitylation of PML.Sci Signal. 2015 Jun 9;8(380):ra56. doi: 10.1126/scisignal.aaa3929. Sci Signal. 2015. PMID: 26060329 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

