Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia
- PMID: 24172896
- PMCID: PMC4133787
- DOI: 10.1038/nature12746
Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia
Abstract
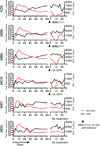
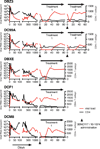
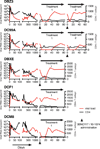
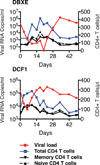
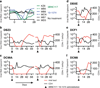
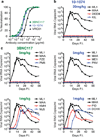
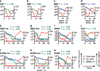
Neutralizing antibodies can confer immunity to primate lentiviruses by blocking infection in macaque models of AIDS. However, earlier studies of anti-human immunodeficiency virus type 1 (HIV-1) neutralizing antibodies administered to infected individuals or humanized mice reported poor control of virus replication and the rapid emergence of resistant variants. A new generation of anti-HIV-1 monoclonal antibodies, possessing extraordinary potency and breadth of neutralizing activity, has recently been isolated from infected individuals. These neutralizing antibodies target different regions of the HIV-1 envelope glycoprotein including the CD4-binding site, glycans located in the V1/V2, V3 and V4 regions, and the membrane proximal external region of gp41 (refs 9-14). Here we have examined two of the new antibodies, directed to the CD4-binding site and the V3 region (3BNC117 and 10-1074, respectively), for their ability to block infection and suppress viraemia in macaques infected with the R5 tropic simian-human immunodeficiency virus (SHIV)-AD8, which emulates many of the pathogenic and immunogenic properties of HIV-1 during infections of rhesus macaques. Either antibody alone can potently block virus acquisition. When administered individually to recently infected macaques, the 10-1074 antibody caused a rapid decline in virus load to undetectable levels for 4-7 days, followed by virus rebound during which neutralization-resistant variants became detectable. When administered together, a single treatment rapidly suppressed plasma viraemia for 3-5 weeks in some long-term chronically SHIV-infected animals with low CD4(+) T-cell levels. A second cycle of anti-HIV-1 monoclonal antibody therapy, administered to two previously treated animals, successfully controlled virus rebound. These results indicate that immunotherapy or a combination of immunotherapy plus conventional antiretroviral drugs might be useful as a treatment for chronically HIV-1-infected individuals experiencing immune dysfunction.
Figures



Comment in
-
HIV: Antibodies advance the search for a cure.Nature. 2013 Nov 14;503(7475):207-8. doi: 10.1038/nature12703. Epub 2013 Oct 30. Nature. 2013. PMID: 24172894 No abstract available.
-
HIV: advancing the antibody approach.Nat Rev Immunol. 2013 Dec;13(12):846-7. doi: 10.1038/nri3573. Nat Rev Immunol. 2013. PMID: 24409525 No abstract available.
Similar articles
-
Early antibody therapy can induce long-lasting immunity to SHIV.Nature. 2017 Mar 23;543(7646):559-563. doi: 10.1038/nature21435. Epub 2017 Mar 13. Nature. 2017. PMID: 28289286 Free PMC article.
-
Development of Broadly Neutralizing Antibodies and Their Mapping by Monomeric gp120 in Human Immunodeficiency Virus Type 1-Infected Humans and Simian-Human Immunodeficiency Virus SHIVSF162P3N-Infected Macaques.J Virol. 2016 Mar 28;90(8):4017-4031. doi: 10.1128/JVI.02898-15. Print 2016 Apr. J Virol. 2016. PMID: 26842476 Free PMC article.
-
Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys.Nature. 2013 Nov 14;503(7475):224-8. doi: 10.1038/nature12744. Epub 2013 Oct 30. Nature. 2013. PMID: 24172905 Free PMC article.
-
Protection of neonatal macaques against experimental SHIV infection by human neutralizing monoclonal antibodies.Transfus Clin Biol. 2001 Aug;8(4):350-8. doi: 10.1016/s1246-7820(01)00187-2. Transfus Clin Biol. 2001. PMID: 11642027 Review.
-
Broadly neutralizing anti-HIV-1 monoclonal antibodies in the clinic.Nat Med. 2019 Apr;25(4):547-553. doi: 10.1038/s41591-019-0412-8. Epub 2019 Apr 1. Nat Med. 2019. PMID: 30936546 Free PMC article. Review.
Cited by
-
Viremic HIV Controllers Exhibit High Plasmacytoid Dendritic Cell-Reactive Opsonophagocytic IgG Antibody Responses against HIV-1 p24 Associated with Greater Antibody Isotype Diversification.J Immunol. 2015 Jun 1;194(11):5320-8. doi: 10.4049/jimmunol.1402918. Epub 2015 Apr 24. J Immunol. 2015. PMID: 25911748 Free PMC article.
-
Broadly neutralizing antibodies potently inhibit cell-to-cell transmission of semen leukocyte-derived SHIV162P3.EBioMedicine. 2020 Jul;57:102842. doi: 10.1016/j.ebiom.2020.102842. Epub 2020 Jun 30. EBioMedicine. 2020. PMID: 32619962 Free PMC article.
-
A Chimeric HIV-1 gp120 Fused with Vaccinia Virus 14K (A27) Protein as an HIV Immunogen.PLoS One. 2015 Jul 24;10(7):e0133595. doi: 10.1371/journal.pone.0133595. eCollection 2015. PLoS One. 2015. PMID: 26208356 Free PMC article.
-
Antibody engineering for increased potency, breadth and half-life.Curr Opin HIV AIDS. 2015 May;10(3):151-9. doi: 10.1097/COH.0000000000000148. Curr Opin HIV AIDS. 2015. PMID: 25760931 Free PMC article. Review.
-
HIV broadly neutralizing antibody targets.Curr Opin HIV AIDS. 2015 May;10(3):135-43. doi: 10.1097/COH.0000000000000153. Curr Opin HIV AIDS. 2015. PMID: 25760932 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

