C9orf72 frontotemporal lobar degeneration is characterised by frequent neuronal sense and antisense RNA foci
- PMID: 24170096
- PMCID: PMC3830745
- DOI: 10.1007/s00401-013-1200-z
C9orf72 frontotemporal lobar degeneration is characterised by frequent neuronal sense and antisense RNA foci
Abstract
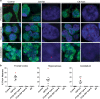
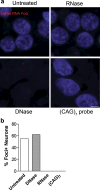
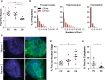
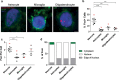
An expanded GGGGCC repeat in a non-coding region of the C9orf72 gene is a common cause of frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis. Non-coding repeat expansions may cause disease by reducing the expression level of the gene they reside in, by producing toxic aggregates of repeat RNA termed RNA foci, or by producing toxic proteins generated by repeat-associated non-ATG translation. We present the first definitive report of C9orf72 repeat sense and antisense RNA foci using a series of C9FTLD cases, and neurodegenerative disease and normal controls. A sensitive and specific fluorescence in situ hybridisation protocol was combined with protein immunostaining to show that both sense and antisense foci were frequent, specific to C9FTLD, and present in neurons of the frontal cortex, hippocampus and cerebellum. High-resolution imaging also allowed accurate analyses of foci number and subcellular localisation. RNA foci were most abundant in the frontal cortex, where 51 % of neurons contained foci. RNA foci also occurred in astrocytes, microglia and oligodendrocytes but to a lesser degree than in neurons. RNA foci were observed in both TDP-43- and p62-inclusion bearing neurons, but not at a greater frequency than expected by chance. RNA foci abundance in the frontal cortex showed a significant inverse correlation with age at onset of disease. These data establish that sense and antisense C9orf72 repeat RNA foci are a consistent and specific feature of C9FTLD, providing new insight into the pathogenesis of C9FTLD.
Figures
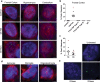
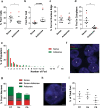
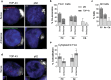
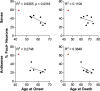
Comment in
-
Making sense of the antisense transcripts in C9FTD/ALS.Acta Neuropathol. 2013 Dec;126(6):785-7. doi: 10.1007/s00401-013-1201-y. Acta Neuropathol. 2013. PMID: 24178412 Free PMC article. No abstract available.
Similar articles
-
Bidirectional nucleolar dysfunction in C9orf72 frontotemporal lobar degeneration.Acta Neuropathol Commun. 2017 Apr 18;5(1):29. doi: 10.1186/s40478-017-0432-x. Acta Neuropathol Commun. 2017. PMID: 28420437 Free PMC article.
-
Antisense RNA foci in the motor neurons of C9ORF72-ALS patients are associated with TDP-43 proteinopathy.Acta Neuropathol. 2015 Jul;130(1):63-75. doi: 10.1007/s00401-015-1429-9. Epub 2015 May 6. Acta Neuropathol. 2015. PMID: 25943887 Free PMC article.
-
Drosha inclusions are new components of dipeptide-repeat protein aggregates in FTLD-TDP and ALS C9orf72 expansion cases.J Neuropathol Exp Neurol. 2015 Apr;74(4):380-7. doi: 10.1097/NEN.0000000000000182. J Neuropathol Exp Neurol. 2015. PMID: 25756586 Free PMC article.
-
Mechanisms of toxicity in C9FTLD/ALS.Acta Neuropathol. 2014 Mar;127(3):359-76. doi: 10.1007/s00401-013-1237-z. Epub 2014 Jan 7. Acta Neuropathol. 2014. PMID: 24394885 Free PMC article. Review.
-
The neuropathology associated with repeat expansions in the C9ORF72 gene.Acta Neuropathol. 2014 Mar;127(3):347-57. doi: 10.1007/s00401-013-1232-4. Epub 2013 Dec 20. Acta Neuropathol. 2014. PMID: 24356984 Review.
Cited by
-
C9ORF72 GGGGCC Expanded Repeats Produce Splicing Dysregulation which Correlates with Disease Severity in Amyotrophic Lateral Sclerosis.PLoS One. 2015 May 27;10(5):e0127376. doi: 10.1371/journal.pone.0127376. eCollection 2015. PLoS One. 2015. PMID: 26016851 Free PMC article.
-
ALS Genetics: Gains, Losses, and Implications for Future Therapies.Neuron. 2020 Dec 9;108(5):822-842. doi: 10.1016/j.neuron.2020.08.022. Epub 2020 Sep 14. Neuron. 2020. PMID: 32931756 Free PMC article. Review.
-
Approaches to Gene Modulation Therapy for ALS.Neurotherapeutics. 2022 Jul;19(4):1159-1179. doi: 10.1007/s13311-022-01285-w. Epub 2022 Sep 6. Neurotherapeutics. 2022. PMID: 36068427 Free PMC article. Review.
-
C9orf72 deficiency promotes microglial-mediated synaptic loss in aging and amyloid accumulation.Neuron. 2021 Jul 21;109(14):2275-2291.e8. doi: 10.1016/j.neuron.2021.05.020. Epub 2021 Jun 15. Neuron. 2021. PMID: 34133945 Free PMC article.
-
The role of inflammation in neurodegeneration: novel insights into the role of the immune system in C9orf72 HRE-mediated ALS/FTD.Mol Neurodegener. 2022 Mar 18;17(1):22. doi: 10.1186/s13024-022-00525-z. Mol Neurodegener. 2022. PMID: 35303907 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

